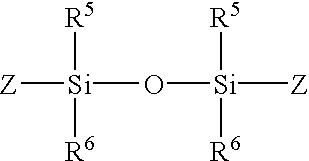US20030048411A1 - Intraoccular lenses capable of in vivo power adjustment and method for same - Google Patents
Intraoccular lenses capable of in vivo power adjustment and method for same Download PDFInfo
- Publication number
- US20030048411A1 US20030048411A1 US10/192,017 US19201702A US2003048411A1 US 20030048411 A1 US20030048411 A1 US 20030048411A1 US 19201702 A US19201702 A US 19201702A US 2003048411 A1 US2003048411 A1 US 2003048411A1
- Authority
- US
- United States
- Prior art keywords
- implant
- optical
- stimulus
- optical element
- exposing
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000000034 method Methods 0.000 title claims abstract description 66
- 238000001727 in vivo Methods 0.000 title claims description 17
- 230000003287 optical effect Effects 0.000 claims abstract description 104
- 239000007943 implant Substances 0.000 claims abstract description 84
- 230000008859 change Effects 0.000 claims abstract description 26
- 238000012360 testing method Methods 0.000 claims abstract description 24
- 229920000642 polymer Polymers 0.000 claims description 53
- 239000011159 matrix material Substances 0.000 claims description 50
- 238000006116 polymerization reaction Methods 0.000 claims description 26
- 239000000203 mixture Substances 0.000 claims description 19
- -1 polysiloxane Polymers 0.000 claims description 18
- 241000283973 Oryctolagus cuniculus Species 0.000 claims description 15
- 230000001988 toxicity Effects 0.000 claims description 5
- 231100000419 toxicity Toxicity 0.000 claims description 5
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 claims description 5
- 229920002554 vinyl polymer Polymers 0.000 claims description 5
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 claims description 4
- 229920001296 polysiloxane Polymers 0.000 claims description 4
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 claims description 3
- KPUWHANPEXNPJT-UHFFFAOYSA-N disiloxane Chemical class [SiH3]O[SiH3] KPUWHANPEXNPJT-UHFFFAOYSA-N 0.000 claims description 3
- 230000001939 inductive effect Effects 0.000 claims description 3
- GKTNLYAAZKKMTQ-UHFFFAOYSA-N n-[bis(dimethylamino)phosphinimyl]-n-methylmethanamine Chemical compound CN(C)P(=N)(N(C)C)N(C)C GKTNLYAAZKKMTQ-UHFFFAOYSA-N 0.000 claims 1
- 230000001954 sterilising effect Effects 0.000 claims 1
- 239000000178 monomer Substances 0.000 description 26
- 230000015572 biosynthetic process Effects 0.000 description 10
- 238000002513 implantation Methods 0.000 description 10
- 239000007858 starting material Substances 0.000 description 10
- 229920001577 copolymer Polymers 0.000 description 9
- 230000004438 eyesight Effects 0.000 description 6
- 206010061218 Inflammation Diseases 0.000 description 5
- 238000011156 evaluation Methods 0.000 description 5
- 230000006870 function Effects 0.000 description 5
- 230000004054 inflammatory process Effects 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 230000029663 wound healing Effects 0.000 description 5
- 0 [1*][Si]([2*])(C)OC.[1*][Si]([2*])(C)O[Si]([3*])([4*])OC Chemical compound [1*][Si]([2*])(C)OC.[1*][Si]([2*])(C)O[Si]([3*])([4*])OC 0.000 description 4
- 125000003118 aryl group Chemical group 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 229920001519 homopolymer Polymers 0.000 description 4
- 208000015181 infectious disease Diseases 0.000 description 4
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- DFDFDKNZJNJCTD-UHFFFAOYSA-N 3-dimethylsilylpropyl 2-methylprop-2-enoate Chemical group C[SiH](C)CCCOC(=O)C(C)=C DFDFDKNZJNJCTD-UHFFFAOYSA-N 0.000 description 3
- 208000002177 Cataract Diseases 0.000 description 3
- 239000004205 dimethyl polysiloxane Substances 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 3
- 238000010561 standard procedure Methods 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- ITMCEJHCFYSIIV-UHFFFAOYSA-N triflic acid Chemical compound OS(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-N 0.000 description 3
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- 241000282887 Suidae Species 0.000 description 2
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 125000000217 alkyl group Chemical group 0.000 description 2
- 125000005336 allyloxy group Chemical group 0.000 description 2
- 238000012937 correction Methods 0.000 description 2
- 238000004132 cross linking Methods 0.000 description 2
- 238000009792 diffusion process Methods 0.000 description 2
- 235000013870 dimethyl polysiloxane Nutrition 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 150000004820 halides Chemical class 0.000 description 2
- 125000001072 heteroaryl group Chemical group 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 2
- 230000000379 polymerizing effect Effects 0.000 description 2
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 2
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 239000011541 reaction mixture Substances 0.000 description 2
- UPYYGCGKWBXZOW-UHFFFAOYSA-M sodium;(4-acetamidophenyl)-hydroxystibinate Chemical group [Na+].CC(=O)NC1=CC=C([Sb](O)([O-])=O)C=C1 UPYYGCGKWBXZOW-UHFFFAOYSA-M 0.000 description 2
- 125000000008 (C1-C10) alkyl group Chemical group 0.000 description 1
- 229920002818 (Hydroxyethyl)methacrylate Polymers 0.000 description 1
- PIZHFBODNLEQBL-UHFFFAOYSA-N 2,2-diethoxy-1-phenylethanone Chemical compound CCOC(OCC)C(=O)C1=CC=CC=C1 PIZHFBODNLEQBL-UHFFFAOYSA-N 0.000 description 1
- ZINSDZNACWQNRN-UHFFFAOYSA-N 2,4-bis(chloromethyl)-1,3,5-triazine Chemical class ClCC1=NC=NC(CCl)=N1 ZINSDZNACWQNRN-UHFFFAOYSA-N 0.000 description 1
- VHSHLMUCYSAUQU-UHFFFAOYSA-N 2-hydroxypropyl methacrylate Chemical compound CC(O)COC(=O)C(C)=C VHSHLMUCYSAUQU-UHFFFAOYSA-N 0.000 description 1
- BQZJOQXSCSZQPS-UHFFFAOYSA-N 2-methoxy-1,2-diphenylethanone Chemical compound C=1C=CC=CC=1C(OC)C(=O)C1=CC=CC=C1 BQZJOQXSCSZQPS-UHFFFAOYSA-N 0.000 description 1
- MDGWPHCHEKVBDM-UHFFFAOYSA-N 3-[methyl-[3-(2-methylprop-2-enoyloxy)propyl]-trimethylsilyloxysilyl]propyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCC[Si](C)(O[Si](C)(C)C)CCCOC(=O)C(C)=C MDGWPHCHEKVBDM-UHFFFAOYSA-N 0.000 description 1
- GNSFRPWPOGYVLO-UHFFFAOYSA-N 3-hydroxypropyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCCO GNSFRPWPOGYVLO-UHFFFAOYSA-N 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 239000004593 Epoxy Substances 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- WOBHKFSMXKNTIM-UHFFFAOYSA-N Hydroxyethyl methacrylate Chemical compound CC(=C)C(=O)OCCO WOBHKFSMXKNTIM-UHFFFAOYSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 241000282579 Pan Species 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- 206010052428 Wound Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 150000008062 acetophenones Chemical class 0.000 description 1
- 125000003668 acetyloxy group Chemical group [H]C([H])([H])C(=O)O[*] 0.000 description 1
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 1
- 229920006397 acrylic thermoplastic Polymers 0.000 description 1
- 125000005250 alkyl acrylate group Chemical group 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 125000004104 aryloxy group Chemical group 0.000 description 1
- 125000000649 benzylidene group Chemical group [H]C(=[*])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 210000004087 cornea Anatomy 0.000 description 1
- 238000013500 data storage Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 230000036040 emmetropia Effects 0.000 description 1
- JREARPFWSGLDLG-UHFFFAOYSA-N ethenyl(dimethyl)silane Chemical group C[SiH](C)C=C JREARPFWSGLDLG-UHFFFAOYSA-N 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 230000009477 glass transition Effects 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 150000004678 hydrides Chemical class 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 239000003999 initiator Substances 0.000 description 1
- 125000002462 isocyano group Chemical group *[N+]#[C-] 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 238000002386 leaching Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 150000002734 metacrylic acid derivatives Chemical class 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- HMMGMWAXVFQUOA-UHFFFAOYSA-N octamethylcyclotetrasiloxane Chemical compound C[Si]1(C)O[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O1 HMMGMWAXVFQUOA-UHFFFAOYSA-N 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 150000002923 oximes Chemical class 0.000 description 1
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 1
- 229920002338 polyhydroxyethylmethacrylate Polymers 0.000 description 1
- 229920000193 polymethacrylate Polymers 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 230000002980 postoperative effect Effects 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 208000014733 refractive error Diseases 0.000 description 1
- 210000001525 retina Anatomy 0.000 description 1
- 238000007142 ring opening reaction Methods 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- PXQLVRUNWNTZOS-UHFFFAOYSA-N sulfanyl Chemical class [SH] PXQLVRUNWNTZOS-UHFFFAOYSA-N 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- ISXSCDLOGDJUNJ-UHFFFAOYSA-N tert-butyl prop-2-enoate Chemical compound CC(C)(C)OC(=O)C=C ISXSCDLOGDJUNJ-UHFFFAOYSA-N 0.000 description 1
- 230000036962 time dependent Effects 0.000 description 1
- 239000013638 trimer Substances 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B3/00—Simple or compound lenses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/14—Eye parts, e.g. lenses, corneal implants; Implanting instruments specially adapted therefor; Artificial eyes
- A61F2/16—Intraocular lenses
- A61F2/1613—Intraocular lenses having special lens configurations, e.g. multipart lenses; having particular optical properties, e.g. pseudo-accommodative lenses, lenses having aberration corrections, diffractive lenses, lenses for variably absorbing electromagnetic radiation, lenses having variable focus
- A61F2/1624—Intraocular lenses having special lens configurations, e.g. multipart lenses; having particular optical properties, e.g. pseudo-accommodative lenses, lenses having aberration corrections, diffractive lenses, lenses for variably absorbing electromagnetic radiation, lenses having variable focus having adjustable focus; power activated variable focus means, e.g. mechanically or electrically by the ciliary muscle or from the outside
- A61F2/1627—Intraocular lenses having special lens configurations, e.g. multipart lenses; having particular optical properties, e.g. pseudo-accommodative lenses, lenses having aberration corrections, diffractive lenses, lenses for variably absorbing electromagnetic radiation, lenses having variable focus having adjustable focus; power activated variable focus means, e.g. mechanically or electrically by the ciliary muscle or from the outside for changing index of refraction, e.g. by external means or by tilting
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/18—Macromolecular materials obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B1/00—Optical elements characterised by the material of which they are made; Optical coatings for optical elements
- G02B1/04—Optical elements characterised by the material of which they are made; Optical coatings for optical elements made of organic materials, e.g. plastics
- G02B1/041—Lenses
- G02B1/043—Contact lenses
-
- G—PHYSICS
- G02—OPTICS
- G02C—SPECTACLES; SUNGLASSES OR GOGGLES INSOFAR AS THEY HAVE THE SAME FEATURES AS SPECTACLES; CONTACT LENSES
- G02C7/00—Optical parts
- G02C7/02—Lenses; Lens systems ; Methods of designing lenses
- G02C7/04—Contact lenses for the eyes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/14—Eye parts, e.g. lenses, corneal implants; Implanting instruments specially adapted therefor; Artificial eyes
- A61F2/16—Intraocular lenses
- A61F2/1613—Intraocular lenses having special lens configurations, e.g. multipart lenses; having particular optical properties, e.g. pseudo-accommodative lenses, lenses having aberration corrections, diffractive lenses, lenses for variably absorbing electromagnetic radiation, lenses having variable focus
- A61F2/1624—Intraocular lenses having special lens configurations, e.g. multipart lenses; having particular optical properties, e.g. pseudo-accommodative lenses, lenses having aberration corrections, diffractive lenses, lenses for variably absorbing electromagnetic radiation, lenses having variable focus having adjustable focus; power activated variable focus means, e.g. mechanically or electrically by the ciliary muscle or from the outside
- A61F2/1635—Intraocular lenses having special lens configurations, e.g. multipart lenses; having particular optical properties, e.g. pseudo-accommodative lenses, lenses having aberration corrections, diffractive lenses, lenses for variably absorbing electromagnetic radiation, lenses having variable focus having adjustable focus; power activated variable focus means, e.g. mechanically or electrically by the ciliary muscle or from the outside for changing shape
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/16—Materials or treatment for tissue regeneration for reconstruction of eye parts, e.g. intraocular lens, cornea
-
- G—PHYSICS
- G02—OPTICS
- G02C—SPECTACLES; SUNGLASSES OR GOGGLES INSOFAR AS THEY HAVE THE SAME FEATURES AS SPECTACLES; CONTACT LENSES
- G02C2202/00—Generic optical aspects applicable to one or more of the subgroups of G02C7/00
- G02C2202/14—Photorefractive lens material
Definitions
- the present invention is directed to a system and method which relates to a novel method for evaluating optical implants such as intraoccular lenses (IOLs).
- the implants are placed in a non-human test subject and evaluated for biocompatibility and operability.
- the method is useful in the design of novel implants as well as obtaining data concerning the safety and operability of the lenses.
- the method is particularly applicable to novel IOLs whose optical properties can be manipulated after the lens has been implanted.
- Optical implants have long been used to correct vision problems or to affect changes in “subjects” vision.
- the most widely used optical implant in the intraoccular lens (IOL) which is used to replace a patient's natural lens when the lens can no longer function. IOLs are most often used when a patient develops cataracts such that normal vision is impossible.
- optical implant such as IOLs
- the ophthalmologist would estimate such properties as the lens power and shape based on pre-operation examination of the patient's eye. A lens meeting those requirements would then be implanted into the eye after the natural lens was removed. While these procedures are generally successful in restoring sight to a patient, until recently, there was no noninvasive procedure for adjusting the optical properties of the lens. Thus, if the surgeon's estimates were incorrect, the patient might still be required to use spectacles or the like to achieve optimum vision.
- PCT/US99/23728 discloses a novel implant whose optical properties can be adjusted post fabrication by one or more regions of the implant to an external stimulus such as light. These novel implants allow a surgeon to adjust the optical properties of an IOL after it has been implanted.
- the invention is a method that allows for testing of both the safety and operability of optical implants. In one embodiment, it is a method for evaluating the safety and operability of novel IOLs whose optical properties can be manipulated in vivo.
- an optical implant is implanted in a non-human test subject. After allowing for wound healing, the implant is then evaluated for biocompatibility and operability.
- operability we mean adjustment of lens dose.
- this includes manipulating the optical properties in the prescribed manner and then evaluating the implant to set if the desired changes occur and are maintained for the desired time period.
- the method involves implanting an adjustable IOL in a non-human test subject.
- Adjustable IOL we refer to IOLs whose optical properties can be manipulated or adjusted post implantation without resort to invasive procedures. Generally, this is accomplished by preparing an implant which has macromers distributed therein. These macromers respond to external stimuli such as light, causing changes in the optical properties of the implant. A more detailed description of these types of implants can be found in PCT/US 99/23728.
- the optical properties of the lens are manipulated in the prescribed manner. In the preferred method, this is accomplished by exposing the lens to an external stimulus, such as light. Ultraviolet light is most preferred.
- the implants are then evaluated for safety, including biocompatibility, and operability, e.g. whether the desired changes in optical properties have taken place. These tests are done using standard methods such as examining the test subject's eyes for evidence of inflammation and physical examination of the lens implant for changes in optical properties. These evaluations can occur either in vivo or ex vivo.
- FIG. 1 is a graph showing the measured change in dioptor for series of adjustable IOLs after an adjustment to the lenses was made in vivo.
- the method of the invention is a means for evaluating the biocompatibility and operability of adjustable optical implants such as IOLs.
- the method involves placing the adjustable implant in a non-human test subject, allowing wound healing to occur and then determining if the implant poses safety or health risks to the subject.
- the method further comprises evaluating the operability of the lens in vivo. By operability is meant whether the implant can be adjusted in the manner intended.
- optical implant refers to any devices or structure implanted in a living organism for the purpose of changing the optical properties of the organisms eyes. Perhaps the most common optical implant is an intraoccular lens the organisms natural lens when the lens becomes damaged or no longer transmits light.
- adjustable optical implants are those optical implants, such as IOLs whose properties can be manipulated or adjusted post implantation by non-invasive measures.
- the adjustable implants contain macromers which are capable of inducing change in the implant when the implant is exposed to an external stimulus.
- Non-human test subject refers to a test subject, other than a human being into which the optical element is implanted. Typically this includes mammals which exhibit physiological similarities to humans and includes rabbits, dogs, pigs and chimpanzees or other simians. Of these, rabbits and pigs are most preferred.
- the first step of the method involves implanting the optical implant into the test subject.
- this involves first removing the existing lens using any standard technique such as phaecoemulsification. Removal of the lens creates a cavity within the capsular bag into which the IOL is then placed.
- the IOL is inserted using standard techniques such as that described in Clayman, Intraoccular Lens Implantation, 1985.
- the IOL can be formed in situ in sets using a procedure describes in Nishi, O. et al, 23 J. Cataract. Refract. Surg. 1548-53 (1997).
- Safety evaluation includes testing and observations for biocompatibility including absence of infection and resistance to leaching of components and degradation.
- Operability evaluation includes a determination as to whether the implants perform as designed. In the case of implants capable of adjustment or manipulation of function preferably optical properties, this includes attempting adjust or manipulate the optical properties of the implant in vivo and then evaluating the implant to determine whether a change in the implant has occurred and the nature and degree of the change.
- the method of the invention comprises a method for evaluating the safety and operability of IOLs that are capable of adjustment or manipulation of their optical properties by non-evasive success.
- the IOLs contains macromers which, when exposed to an external stimulus, induce changes in the optical properties of the IOL.
- the optical properties of the implant are manipulated by stimulus induced polymerization of the macromers causes changes in the refractive index of the implant, the shape of the implant or both refractive index and shape.
- the preferred stimulus used to induce polymerization of the macromers is light with ultraviolet light most preferred.
- the IOLs are exposed to an external stimulus, such as ultraviolet light, in a prescribed pattern, intensity, and duration.
- the pattern, intensity and duration of the exposure is determined by the desired changes of optical properties and the nature of the macromers and other components dispersed throughout the IOL.
- the IOLs are also examined to determine if the desired change in optical properties has occurred. This can be accomplished in several ways including physical examination of the lens in vivo to see if the desired change in shape has occurred, use of refractive techniques to see if the desired change in refractive has occurred and explantation of the lens followed by examination of the change in optical properties ex vivo. While these are the preferred methods of evaluating the performance of the IOL, other techniques are known.
- optical elements that are capable of post operative adjustment of their properties.
- One class of these elements is optical elements that comprise a first polymer matrix and a macromer dispersed therein.
- the first polymer matrix forms the optical element framework and is generally responsible for many of its material properties.
- the macromer (“macromer”) may be a single compound or a combination of compounds that is capable of stimulus-induced polymerization, preferably photo-polymerization.
- polymerization refers to a reaction wherein at least one of the components of the macromer reacts to form at least one covalent or physical bond with either a like component or with a different component.
- first polymer matrix and the macromers will depend on the end use of the optical element. However, as a general rule, the first polymer matrix and the macromers are selected such that the components that comprise the macromer are capable of diffusion within the first polymer matrix. Put another way, a loose first polymer matrix will tend to be paired with larger macromer components and a tight first polymer matrix will tend to be paired with smaller macromer components.
- the macromer Upon exposure to an appropriate energy source (e.g., heat or light), the macromer typically form a second polymer matrix in the exposed region of the optical element.
- the presence of the second polymer matrix changes the material characteristics of this portion of the optical element to modulate its refraction capabilities.
- the formation of the second polymer matrix typically increases the refractive index of the affected portion of the optical element.
- the macromer in the unexposed region will migrate into the exposed region over time. The amount of macromer migration into the exposed region is time dependent and may be precisely controlled. If enough time is permitted, the macromer components will re-equilibrate and redistribute throughout optical element (i.e., the first polymer matrix, including the exposed region).
- FIG. 1 illustrates one inventive embodiment, refractive index modulation (thus lens power modulation) followed by a lock in.
- the first polymer matrix is a covalently or physically linked structure that functions as an optical element and is formed from a first polymer matrix composition (“FPMC”).
- FPMC first polymer matrix composition
- the first polymer matrix composition comprises one or more monomers that upon polymerization will form the first polymer matrix.
- the first polymer matrix composition optionally may include any number of formulation auxiliaries that modulate the polymerization reaction or improve any property of the optical element.
- suitable FPMC monomers include acrylics, methacrylates, phosphazenes, siloxanes, vinyls, homopolymers and copolymers thereof.
- a “monomer” refers to any unit (which may itself either be a homopolymer or copolymer) which may be linked together to form a polymer containing repeating units of the same. If the FPMC monomer is a copolymer, it may be comprised of the same type of monomers (e.g., two different siloxanes) or it may be comprised of different types of monomers (e.g., a siloxane and an acrylic).
- the one or more monomers that form the first polymer matrix are polymerized and cross-linked in the presence of the macromer.
- polymeric starting material that forms the first polymer matrix is cross-linked in the presence of the macromer.
- the macromer components must be compatible with and not appreciably interfere with the formation of the first polymer matrix.
- the formation of the second polymer matrix should also be compatible with the existing first polymer matrix. Put another way, the first polymer matrix and the second polymer matrix should not phase separate and light transmission by the optical element should be unaffected.
- the macromer may be a single component or multiple components so long as (i) it is compatible with the formation of the first polymer matrix; (ii) it remains capable to stimulus-induced polymerization after the formation of the first polymer matrix; and (iii) it is freely diffusable within the first polymer matrix.
- the stimulus-induced polymerization is photo-induced polymerization.
- inventive optical elements have numerous applications in the electronics and data storage industries.
- Another application for the present invention is as medical lenses, particularly as intraoccular lenses.
- IOLs intraoccular lenses
- the first type of an intraoccular lens replaces the eye's natural lens. The most common reason for such a procedure is cataracts.
- the second type of intraoccular lens supplements the existing lens and functions as a permanent corrective lens.
- This type of lens (sometimes referred to as a phakic intraoccular lens) is implanted in the anterior or posterior chamber to correct any refractive errors of the eye.
- the power for either type of intraoccular lenses required for emmetropia i.e., perfect focus on the retina from light at infinity
- the inventive intraoccular lens comprises a first polymer matrix and a macromer dispersed therein.
- the first polymer matrix and the macromer are as described above with the additional requirement that the resulting lens be biocompatible.
- Illustrative example of a suitable first polymer matrix include: poly-acrylates such as poly-alkyl acrylates and poly-hydroxyalkyl acrylates; poly-methacrylates such as poly-methyl methaacrylate (“PMMA”), poly-hydroxyethyl methacrylate (“PHEMA”), and poly-hydroxypropyl methacrylate (“HPMA”); poly-vinyls such as poly-styrene and poly-vinylpyrrolidone (“PNVP”); poly-siloxanes such as poly-dimethylsiloxane; poly-phosphazenes,and copolymers of thereof.
- PMMA poly-methyl methaacrylate
- PHEMA poly-hydroxyethyl methacrylate
- HPMA poly-hydroxypropyl methacrylate
- poly-vinyls such as poly-styrene and poly-vinylpyrrolidone (“PNVP”
- PNVP poly-siloxanes
- the first polymer matrix generally possesses a relatively low glass transition temperature (“T g ”) such that the resulting IOL tends to exhibit fluid-like and/or elastomeric behavior, and is typically formed by crosslinking one or more polymeric starting materials wherein each polymeric starting material includes at least one crosslinkable group.
- T g glass transition temperature
- suitable crosslinkable groups include but are not limited to hydride, acetoxy, alkoxy, amino, anhydride, aryloxy, carboxy, enoxy, epoxy, halide, isocyano, olefinic, and oxime.
- each polymeric starting material includes terminal monomers (also referred to as endcaps) that are either the same or different from the one or more monomers that comprise the polymeric starting material but include at least one crosslinkable group.
- the terminal monomers begin and end the polymeric starting material and include at least one crosslinkable group as part of its structure.
- the mechanism for crosslinking the polymeric starting material preferably is different than the mechanism for the stimulus-induced polymerization of the components that comprise the Macromer. For example, if the Macromer is polymerized by photo-induced polymerization, then it is preferred that the polymeric starting materials have crosslinkable groups that are polymerized by any mechanism other than photo-induced polymerization.
- An especially preferred class of polymeric starting materials for the formation of the first polymer matrix is poly-siloxanes (also known as “silicones”) endcapped with a terminal monomer which includes a crosslinkable group selected from the group consisting of acetoxy, amino, alkoxy, halide, hydroxy, and mercapto. Because silicone IOLs tend to be flexible and foldable, generally smaller incisions may be used during the IOL implantation procedure.
- An example of an especially preferred polymeric starting material is bis(diacetoxymethylsilyl)-polydimethylsiloxane (which is poly-dimethylsiloxane that is endcapped with a diacetoxymethylsilyl erminal monomer).
- the macromer that is used in fabricating IOLs is as described above except that it has the additional requirement of biocompatibility.
- the macromer is capable of stimulus-induced polymerization and may be a single component or multiple components so long as (i) it is compatible with the formation of the first polymer matrix; (ii) it remains capable of stimulus-induced polymerization after the formation of the first polymer matrix; and (iii) it is freely diffusable within the first polymer matrix.
- the same type of monomers that is used to form the first polymer matrix may be used as a component of the macromer.
- Macromer may include other components such as initiators and sensitizers that facilitate the formation of the second polymer matrix.
- the stimulus-induced polymerization is photo-polymerization.
- the one or more monomers that comprise the macromers each preferably includes at least one group that is capable of photopolymerization.
- Illustrative examples of such photopolymerizable groups include but are not limited to acrylate, allyloxy, cinnamoyl, methacrylate, stibenyl, and vinyl.
- the macromer includes a photoinitiator (any compound used to generate free radicals) ether alone or in the presence of a sensitizer.
- photinitiators examples include acetophenones (e.g., -substituted haloacetophenones, and diethoxyacetophenone); 2,4-dichloromethyl-1,3,5-triazines; benzoin methyl ether; and o-benzoyl oximino ketone.
- suitable sensitizers include p-(dialkylamino)aryl aldehyde; N-alkylindolyidene Check sp; and bis[p-(dialkylamino)benzylidene] ketone.
- an especially preferred class of macromer monomers is poly-siloxanes endcapped with a termination siloxane moiety that includes a photopolymerizable group.
- An illustrative representation of such a monomer is
- adjustable optical implants are those optical implants such as IOLs whose properties can be manipulated or adjusted post implantation by non-invasive measures.
- the adjustable implant's contain macromers which are capable of inducing change in the implant when the implant is exposed to an external stimulus.
- Y is a siloxane which may be a monomer, a homopolymer or a copolymer formed from any number of siloxane units, and X and X 1 may be the same or different and are each independently a terminal siloxane moiety that includes a photopolymerizable group.
- Y include
- R 1 , R 2 , R 3 , and R 4 are independently each hydrogen, alkyl (primary, secondary, tertiary, cyclo), aryl, or heteroaryl.
- R 1 , R 2 , R 3 , and R 4 is a C 1 -C 10 alkyl or phenyl.
- at least one R 1 , R 2 , R 3 , and R 4 is an aryl, particularly phenyl.
- R 1 , R 2 , and R 3 are the same and are methyl, ethyl or propyl and R 4 is phenyl.
- R 5 and R 6 are independently each hydrogen, alkyl, aryl, or heteroaryl
- Z is a photopolymerizable group.
- R 5 and R 6 are independently each a C 1 -C 10 or phenyl and Z is a photopolymerizable group that includes a moiety selected from the group consisting of acrylate, allyloxy, connamoyl, methaacrylate, stibenyl, and vinyl.
- R 5 and R 6 is methyl, ethyl, or propyl and Z is a photopolymerizable group that includes an acrylate or methacrylate moiety.
- an macromer monomer if of the following formula
- Macromer monomers include dimethylsiloxane-diphenylsiloxane copolymer endcapped with a vinyl dimethylsilane group; dimethylsiloxane-methylphenylsiloxane copolymer endcapped with a methacryloxypropyl dimethylsilane group; and dimethylsiloxane endcapped with a methacryloxypropyldimethylsilane group.
- a ring-opening reaction of one or more cyclic siloxanes in the presence of triflic acid has been found to be a particularly efficient method of making one class of inventive macromers. Briefly, the method comprises contacting a cyclic siloxane with a compound of the formula
- the cyclic siloxane may be a cyclic siloxane monomer, homopolymer, or copolymer. Alternatively, more than one cyclic siloxane may be used.
- a cyclic dimethylsiloxane tetramer and a cyclic methyl-phenylsiloxane trimer are contacted with bis-methacryloxypropyltetramethyldisiloxane in the presence of triflic acid to form a dimethyl-siloxane methyl-phenylsiloxane copolymer that is endcapped with a methacryloxypropyl-dimethylsilane group, an especially preferred macromer.
- the adjustable IOLs may be fabricated with any suitable method that results in a first polymer matrix with one or more components which comprise the Macromer dispersed therein, and where the Macromer is capable of stimulus-induced polymerization to form a second polymer matrix.
- the method for making an inventive IOL is the same as that for making an inventive optical element. In one embodiment, the method comprises:
- the type of mold that is used will depend on the optical element being made. For example, if the optical element is a prism, then a mold in the shape of a prism is used. Similarly, if the optical element is an intraoccular lens, then an intraoccular lens mold is used and so forth.
- the first polymer matrix composition comprises one or more monomers for forming the first polymer matrix and optionally includes any number of formulation auxiliaries that either modulate the polymerization reaction or improve any property (whether or not related to the optical characteristic) of the optical element.
- the Macromer comprises one or more components that together are capable of stimulus-induced polymerization to form the second polymer matrix. Because flexible and foldable intraoccular lenses generally permit smaller incisions, it is preferred that both the first polymer matrix composition and the Macromer include one or more silicone-based or low T g acrylic monomers when the inventive method is used to make IOLs.
- the adjustable optical element is then implanted in a non-human test subject. Implantation entails removal of the existing lens by phaecoemulsification and extraction of the lens debries. This is followed by implantation of the element using standard surgical procedures.
- Operability testing involves attempting to manipulate the properties of the implant in vivo followed by an evaluation as to whether the desired changes have occurred.
- this entails exposing at least a portion of the implant to an external stimulus so as to induce a change in the properties of the implant.
- ultraviolet light is said to induce photopolymerization of macromers in at least a portion of an adjustable IOL polymerization of the macromers causes change in the shape of the IOL and/or the refractive index of the IOL. The extent of the changes is then evaluated to see if the desired optical properties have been achieved.
- Determination of biocompatibility can be accomplished either in vivo or ex vivo or both. Physical examination of the eye can be used to determine the presence of inflammation and their biocompatibility. In some cases, however, it may be necessary to explant the lens and conduct histopathological studies of the eye tissue to determine biocompatibility.
- the determination of operability requires that at least the adjustment phase be done in vivo followed by examination of the lens in vivo or ex vivo.
- In vivo examination of the lens can be done using an autoretractometer or a Scheimpflug imaging device to determine change in refraction and/or shape.
- the lens may be explanted after an ajustment lens has been attempted and the changes in the lens can be determined ex vivo.
- Sterilized, adjustable IOLs were implanted in albino rabbit eyes. After clinically following the eyes for one week, the rabbits were sacrificed. The extracted eyes were evaluated, placed in familiar and studied histopathologically. There was no evidence of corneal toxicity, anterior segment inflammation or other signs of lens toxicity.
- a series of adjustable IOLs were prepared for implantation.
- the IOLs comprised a silicon based polymer matrix with dimethylsiloxane macromer dispersed therein.
- the safety and operability of the lenses was evaluated in four rabbits. The rabbits were first anesthetized and the existing lens was removed using phaecoemulsification. The IOLs were then implanted into the rabbits.
- the rabbit eyes were exposed to ultraviolet light for 60 to 120 seconds to induce localized polymerization of macromer in the center of the lens.
- the lenses were then examined to determine if the desired changes in optical properties had taken place. This was accomplished by explanting the lenses and then evaluating the change in lens power achieved. In this case the power of the lenses increased an average of 0.72 diopters.
Abstract
Description
- This is a continuation-in-part of U.S. application Ser. No. 09/416,044 filed Oct. 8, 1999; which claims priority of U.S. Provisional Application No. 60/115,617 filed Jan. 12, 1999; claims priority of U.S. Provisional Application No. 60/132,871 filed May 5, 1999; and claims priority of U.S. Provisional Application No. 60/140,298 filed Jun. 17, 1999; and which is fully incorporated herein by reference.
- The present invention is directed to a system and method which relates to a novel method for evaluating optical implants such as intraoccular lenses (IOLs). In the present invention, the implants are placed in a non-human test subject and evaluated for biocompatibility and operability. The method is useful in the design of novel implants as well as obtaining data concerning the safety and operability of the lenses. The method is particularly applicable to novel IOLs whose optical properties can be manipulated after the lens has been implanted.
- Optical implants have long been used to correct vision problems or to affect changes in “subjects” vision. The most widely used optical implant in the intraoccular lens (IOL) which is used to replace a patient's natural lens when the lens can no longer function. IOLs are most often used when a patient develops cataracts such that normal vision is impossible.
- Until recently, the optical properties of optical implant such as IOLs were predetermined prior to implantation. In the case of IOLs, the ophthalmologist would estimate such properties as the lens power and shape based on pre-operation examination of the patient's eye. A lens meeting those requirements would then be implanted into the eye after the natural lens was removed. While these procedures are generally successful in restoring sight to a patient, until recently, there was no noninvasive procedure for adjusting the optical properties of the lens. Thus, if the surgeon's estimates were incorrect, the patient might still be required to use spectacles or the like to achieve optimum vision.
- Recently, a novel type of optical implant has been developed which allows for manipulation of the optical properties of the implant after it has been implanted in the patient. PCT/US99/23728 discloses a novel implant whose optical properties can be adjusted post fabrication by one or more regions of the implant to an external stimulus such as light. These novel implants allow a surgeon to adjust the optical properties of an IOL after it has been implanted.
- The invention is a method that allows for testing of both the safety and operability of optical implants. In one embodiment, it is a method for evaluating the safety and operability of novel IOLs whose optical properties can be manipulated in vivo.
- In practice of the invention, an optical implant is implanted in a non-human test subject. After allowing for wound healing, the implant is then evaluated for biocompatibility and operability. By operability, we mean adjustment of lens dose. In the case of implants which are capable of post implant manipulation of optical properties, this includes manipulating the optical properties in the prescribed manner and then evaluating the implant to set if the desired changes occur and are maintained for the desired time period.
- In a preferred embodiment, the method involves implanting an adjustable IOL in a non-human test subject. By the term “Adjustable IOL” we refer to IOLs whose optical properties can be manipulated or adjusted post implantation without resort to invasive procedures. Generally, this is accomplished by preparing an implant which has macromers distributed therein. These macromers respond to external stimuli such as light, causing changes in the optical properties of the implant. A more detailed description of these types of implants can be found in PCT/US 99/23728.
- After the implant has been placed in the test subject, and following wound healing, the optical properties of the lens are manipulated in the prescribed manner. In the preferred method, this is accomplished by exposing the lens to an external stimulus, such as light. Ultraviolet light is most preferred.
- After the optical properties have been manipulated in the desired manner, the implants are then evaluated for safety, including biocompatibility, and operability, e.g. whether the desired changes in optical properties have taken place. These tests are done using standard methods such as examining the test subject's eyes for evidence of inflammation and physical examination of the lens implant for changes in optical properties. These evaluations can occur either in vivo or ex vivo.
- The foregoing has outlined rather broadly the features and technical advantages of the present invention in order that the detailed description of the invention that follows may be better understood. Additional features and advantages of the invention will be described hereinafter which form the subject of the claims of the invention. It should be appreciated by those skilled in the art that the conception and specific embodiment disclosed may be readily utilized as a basis for modifying or designing other structures for carrying out the same purposes of the present invention. It should also be realized by those skilled in the art that such equivalent constructions do not depart from the spirit and scope of the invention as set forth in the appended claims. The novel features which are believed to be characteristic of the invention, both as to its organization and method of operation, together with further objects and advantages will be better understood from the following description when considered in connection with the accompanying figures. It is to be expressly understood, however, that each of the figures is provided for the purpose of illustration and description only and is not intended as a definition of the limits of the present invention.
- For a more complete understanding of the present invention, reference is now made to the following descriptions taken in conjunction with the accompanying drawing, in which:
- FIG. 1 is a graph showing the measured change in dioptor for series of adjustable IOLs after an adjustment to the lenses was made in vivo.
- The method of the invention is a means for evaluating the biocompatibility and operability of adjustable optical implants such as IOLs. The method involves placing the adjustable implant in a non-human test subject, allowing wound healing to occur and then determining if the implant poses safety or health risks to the subject. The method further comprises evaluating the operability of the lens in vivo. By operability is meant whether the implant can be adjusted in the manner intended.
- The term optical implant refers to any devices or structure implanted in a living organism for the purpose of changing the optical properties of the organisms eyes. Perhaps the most common optical implant is an intraoccular lens the organisms natural lens when the lens becomes damaged or no longer transmits light. As described more fully below, adjustable optical implants are those optical implants, such as IOLs whose properties can be manipulated or adjusted post implantation by non-invasive measures. In the preferred embodiment, the adjustable implants contain macromers which are capable of inducing change in the implant when the implant is exposed to an external stimulus.
- Non-human test subject refers to a test subject, other than a human being into which the optical element is implanted. Typically this includes mammals which exhibit physiological similarities to humans and includes rabbits, dogs, pigs and chimpanzees or other simians. Of these, rabbits and pigs are most preferred.
- The first step of the method involves implanting the optical implant into the test subject. In the case of an IOL this involves first removing the existing lens using any standard technique such as phaecoemulsification. Removal of the lens creates a cavity within the capsular bag into which the IOL is then placed. The IOL is inserted using standard techniques such as that described in Clayman, Intraoccular Lens Implantation, 1985. Alternatively, the IOL can be formed in situ in sets using a procedure describes in Nishi, O. et al, 23 J. Cataract. Refract. Surg. 1548-53 (1997).
- Once the implant is in place and the incision closed, the wound created by the placement of the implant is allowed to heal. This typically takes from 7 to 14 days.
- Following wound healing the optical implants are then evaluated for safety and if desired, operability. Safety evaluation includes testing and observations for biocompatibility including absence of infection and resistance to leaching of components and degradation. Operability evaluation includes a determination as to whether the implants perform as designed. In the case of implants capable of adjustment or manipulation of function preferably optical properties, this includes attempting adjust or manipulate the optical properties of the implant in vivo and then evaluating the implant to determine whether a change in the implant has occurred and the nature and degree of the change.
- In the preferred embodiment, the method of the invention comprises a method for evaluating the safety and operability of IOLs that are capable of adjustment or manipulation of their optical properties by non-evasive success. In one embodiment the IOLs contains macromers which, when exposed to an external stimulus, induce changes in the optical properties of the IOL. In the most preferred embodiment, the optical properties of the implant are manipulated by stimulus induced polymerization of the macromers causes changes in the refractive index of the implant, the shape of the implant or both refractive index and shape. The preferred stimulus used to induce polymerization of the macromers is light with ultraviolet light most preferred.
- To determine the operability of the IOLs after implanting them in the test subject, the IOLs are exposed to an external stimulus, such as ultraviolet light, in a prescribed pattern, intensity, and duration. The pattern, intensity and duration of the exposure is determined by the desired changes of optical properties and the nature of the macromers and other components dispersed throughout the IOL. Once the IOL has been exposed to the external stimulus, it is left in the eye for further evaluation of the IOLs safety and biocompatibility. This is done by observing the eye for signs of infection, migration of material from the IOL and the like which are indicator, that the lens is not biocompatible.
- The IOLs are also examined to determine if the desired change in optical properties has occurred. This can be accomplished in several ways including physical examination of the lens in vivo to see if the desired change in shape has occurred, use of refractive techniques to see if the desired change in refractive has occurred and explantation of the lens followed by examination of the change in optical properties ex vivo. While these are the preferred methods of evaluating the performance of the IOL, other techniques are known.
- One type of optical element which can be evaluated using the novel method described herein are optical elements that are capable of post operative adjustment of their properties. One class of these elements is optical elements that comprise a first polymer matrix and a macromer dispersed therein. The first polymer matrix forms the optical element framework and is generally responsible for many of its material properties. The macromer (“macromer”) may be a single compound or a combination of compounds that is capable of stimulus-induced polymerization, preferably photo-polymerization. As used herein, the term “polymerization” refers to a reaction wherein at least one of the components of the macromer reacts to form at least one covalent or physical bond with either a like component or with a different component. The identities of the first polymer matrix and the macromers will depend on the end use of the optical element. However, as a general rule, the first polymer matrix and the macromers are selected such that the components that comprise the macromer are capable of diffusion within the first polymer matrix. Put another way, a loose first polymer matrix will tend to be paired with larger macromer components and a tight first polymer matrix will tend to be paired with smaller macromer components.
- Upon exposure to an appropriate energy source (e.g., heat or light), the macromer typically form a second polymer matrix in the exposed region of the optical element. The presence of the second polymer matrix changes the material characteristics of this portion of the optical element to modulate its refraction capabilities. In general, the formation of the second polymer matrix typically increases the refractive index of the affected portion of the optical element. After exposure, the macromer in the unexposed region will migrate into the exposed region over time. The amount of macromer migration into the exposed region is time dependent and may be precisely controlled. If enough time is permitted, the macromer components will re-equilibrate and redistribute throughout optical element (i.e., the first polymer matrix, including the exposed region). When the region is re-exposed to the energy source, the macromer (“macromer”) that has since migrated into the region (which may be less than if the macromer composition were allowed to re-equilibrate) polymerizes to further increase the formation of the second polymer matrix. This process (exposure followed by an appropriate time interval to allow for diffusion) may be repeated until the exposed region of the optical element has reached the desired property (e.g., power, refractive index, or shape). At this point, the entire optical element is exposed to the energy source to “lock-in” the desired lens property by polymerizing the remaining macromer components that are outside the exposed region before the components can migrate into the exposed region. In other words, because freely diffusable macromer components are no longer available, subsequent exposure of the optical element to an energy source cannot further change its power. FIG. 1 illustrates one inventive embodiment, refractive index modulation (thus lens power modulation) followed by a lock in.
- The first polymer matrix is a covalently or physically linked structure that functions as an optical element and is formed from a first polymer matrix composition (“FPMC”). In general, the first polymer matrix composition comprises one or more monomers that upon polymerization will form the first polymer matrix. The first polymer matrix composition optionally may include any number of formulation auxiliaries that modulate the polymerization reaction or improve any property of the optical element. Illustrative examples of suitable FPMC monomers include acrylics, methacrylates, phosphazenes, siloxanes, vinyls, homopolymers and copolymers thereof. As used herein, a “monomer” refers to any unit (which may itself either be a homopolymer or copolymer) which may be linked together to form a polymer containing repeating units of the same. If the FPMC monomer is a copolymer, it may be comprised of the same type of monomers (e.g., two different siloxanes) or it may be comprised of different types of monomers (e.g., a siloxane and an acrylic).
- In one embodiment, the one or more monomers that form the first polymer matrix are polymerized and cross-linked in the presence of the macromer. In another embodiment, polymeric starting material that forms the first polymer matrix is cross-linked in the presence of the macromer. Under either scenario the macromer components must be compatible with and not appreciably interfere with the formation of the first polymer matrix. Similarly, the formation of the second polymer matrix should also be compatible with the existing first polymer matrix. Put another way, the first polymer matrix and the second polymer matrix should not phase separate and light transmission by the optical element should be unaffected.
- As described previously, the macromer may be a single component or multiple components so long as (i) it is compatible with the formation of the first polymer matrix; (ii) it remains capable to stimulus-induced polymerization after the formation of the first polymer matrix; and (iii) it is freely diffusable within the first polymer matrix. In preferred embodiments, the stimulus-induced polymerization is photo-induced polymerization.
- The inventive optical elements have numerous applications in the electronics and data storage industries. Another application for the present invention is as medical lenses, particularly as intraoccular lenses.
- In general, there are two types of intraoccular lenses (“IOLs”). The first type of an intraoccular lens replaces the eye's natural lens. The most common reason for such a procedure is cataracts. The second type of intraoccular lens supplements the existing lens and functions as a permanent corrective lens. This type of lens (sometimes referred to as a phakic intraoccular lens) is implanted in the anterior or posterior chamber to correct any refractive errors of the eye. In theory, the power for either type of intraoccular lenses required for emmetropia (i.e., perfect focus on the retina from light at infinity) can be precisely calculated. However, in practice, due to errors in measurement of corneal curvature, and/or variable lens positioning and wound healing, it is estimated that only about half of all patients undergoing IOL implantation will enjoy the best possible vision without the need for additional correction after surgery. Because prior art IOLs are generally incapable of post-surgical power modification, the remaining patients must resort to other types of vision correction such as external lenses (e.g., glasses or contact lenses) or cornea surgery. The need for these types of additional corrective measures is obviated with the use of the intraoccular lenses of the present invention.
- The inventive intraoccular lens comprises a first polymer matrix and a macromer dispersed therein. The first polymer matrix and the macromer are as described above with the additional requirement that the resulting lens be biocompatible.
- Illustrative example of a suitable first polymer matrix include: poly-acrylates such as poly-alkyl acrylates and poly-hydroxyalkyl acrylates; poly-methacrylates such as poly-methyl methaacrylate (“PMMA”), poly-hydroxyethyl methacrylate (“PHEMA”), and poly-hydroxypropyl methacrylate (“HPMA”); poly-vinyls such as poly-styrene and poly-vinylpyrrolidone (“PNVP”); poly-siloxanes such as poly-dimethylsiloxane; poly-phosphazenes,and copolymers of thereof. U.S. Pat. No. 4,260,725 and patents and references cited therein (which we all incorporated herein by reference) provide more specific examples of suitable polymers that may be used to form the first polymer matrix.
- In preferred embodiments, the first polymer matrix generally possesses a relatively low glass transition temperature (“T g”) such that the resulting IOL tends to exhibit fluid-like and/or elastomeric behavior, and is typically formed by crosslinking one or more polymeric starting materials wherein each polymeric starting material includes at least one crosslinkable group. Illustrative examples of suitable crosslinkable groups include but are not limited to hydride, acetoxy, alkoxy, amino, anhydride, aryloxy, carboxy, enoxy, epoxy, halide, isocyano, olefinic, and oxime. In more preferred embodiments, each polymeric starting material includes terminal monomers (also referred to as endcaps) that are either the same or different from the one or more monomers that comprise the polymeric starting material but include at least one crosslinkable group. In other words, the terminal monomers begin and end the polymeric starting material and include at least one crosslinkable group as part of its structure. Although it is not necessary for the practice of the present invention, the mechanism for crosslinking the polymeric starting material preferably is different than the mechanism for the stimulus-induced polymerization of the components that comprise the Macromer. For example, if the Macromer is polymerized by photo-induced polymerization, then it is preferred that the polymeric starting materials have crosslinkable groups that are polymerized by any mechanism other than photo-induced polymerization.
- An especially preferred class of polymeric starting materials for the formation of the first polymer matrix is poly-siloxanes (also known as “silicones”) endcapped with a terminal monomer which includes a crosslinkable group selected from the group consisting of acetoxy, amino, alkoxy, halide, hydroxy, and mercapto. Because silicone IOLs tend to be flexible and foldable, generally smaller incisions may be used during the IOL implantation procedure. An example of an especially preferred polymeric starting material is bis(diacetoxymethylsilyl)-polydimethylsiloxane (which is poly-dimethylsiloxane that is endcapped with a diacetoxymethylsilyl erminal monomer).
- The macromer that is used in fabricating IOLs is as described above except that it has the additional requirement of biocompatibility. The macromer is capable of stimulus-induced polymerization and may be a single component or multiple components so long as (i) it is compatible with the formation of the first polymer matrix; (ii) it remains capable of stimulus-induced polymerization after the formation of the first polymer matrix; and (iii) it is freely diffusable within the first polymer matrix. In general, the same type of monomers that is used to form the first polymer matrix may be used as a component of the macromer. However, because of the requirement that the macromer monomers must be diffusable within the first polymer matrix, the macromers generally tend to be smaller (i.e., have lower molecular weights) than the monomers which form the first polymer matrix. In addition to the one or more monomers, Macromer may include other components such as initiators and sensitizers that facilitate the formation of the second polymer matrix.
- In preferred embodiments, the stimulus-induced polymerization is photo-polymerization. In other words, the one or more monomers that comprise the macromers each preferably includes at least one group that is capable of photopolymerization. Illustrative examples of such photopolymerizable groups include but are not limited to acrylate, allyloxy, cinnamoyl, methacrylate, stibenyl, and vinyl. In more preferred embodiments, the macromer includes a photoinitiator (any compound used to generate free radicals) ether alone or in the presence of a sensitizer. Examples of suitable photinitiators include acetophenones (e.g., -substituted haloacetophenones, and diethoxyacetophenone); 2,4-dichloromethyl-1,3,5-triazines; benzoin methyl ether; and o-benzoyl oximino ketone. Examples of suitable sensitizers include p-(dialkylamino)aryl aldehyde; N-alkylindolyidene Check sp; and bis[p-(dialkylamino)benzylidene] ketone.
- Because of the preference for flexible and foldable IOLs, an especially preferred class of macromer monomers is poly-siloxanes endcapped with a termination siloxane moiety that includes a photopolymerizable group. An illustrative representation of such a monomer is
- X—Y—X1
- As described more fully below, adjustable optical implants are those optical implants such as IOLs whose properties can be manipulated or adjusted post implantation by non-invasive measures. In the preferred embodiment, the adjustable implant's contain macromers which are capable of inducing change in the implant when the implant is exposed to an external stimulus.
-
- wherein m and n are independently each an integer and R 1, R2, R3, and R4 are independently each hydrogen, alkyl (primary, secondary, tertiary, cyclo), aryl, or heteroaryl. In preferred embodiments, R1, R2, R3, and R4 is a C1-C10 alkyl or phenyl. Because Macromer monomers with a relatively high aryl content have been found to produce larger changes in the refractive index of the inventive lens, it is generally preferred that at least one R1, R2, R3, and R4 is an aryl, particularly phenyl. In more preferred embodiments, R1, R2, and R3 are the same and are methyl, ethyl or propyl and R4 is phenyl.
-
- Respectively wherein:
- R 5 and R6 are independently each hydrogen, alkyl, aryl, or heteroaryl; and
- Z is a photopolymerizable group.
- In preferred embodiments, R 5 and R6 are independently each a C1-C10 or phenyl and Z is a photopolymerizable group that includes a moiety selected from the group consisting of acrylate, allyloxy, connamoyl, methaacrylate, stibenyl, and vinyl. In more preferred embodiments, R5 and R6 is methyl, ethyl, or propyl and Z is a photopolymerizable group that includes an acrylate or methacrylate moiety.
-
- wherein X and X 1 are the same and R1, R2, R3, and R4 are as defined previously. Illustrative examples of such Macromer monomers include dimethylsiloxane-diphenylsiloxane copolymer endcapped with a vinyl dimethylsilane group; dimethylsiloxane-methylphenylsiloxane copolymer endcapped with a methacryloxypropyl dimethylsilane group; and dimethylsiloxane endcapped with a methacryloxypropyldimethylsilane group. Although any suitable method may be used, a ring-opening reaction of one or more cyclic siloxanes in the presence of triflic acid has been found to be a particularly efficient method of making one class of inventive macromers. Briefly, the method comprises contacting a cyclic siloxane with a compound of the formula
- in the presence of triflic acid wherein R 5, R6, and Z are as defined previously. The cyclic siloxane may be a cyclic siloxane monomer, homopolymer, or copolymer. Alternatively, more than one cyclic siloxane may be used. For example, a cyclic dimethylsiloxane tetramer and a cyclic methyl-phenylsiloxane trimer are contacted with bis-methacryloxypropyltetramethyldisiloxane in the presence of triflic acid to form a dimethyl-siloxane methyl-phenylsiloxane copolymer that is endcapped with a methacryloxypropyl-dimethylsilane group, an especially preferred macromer.
- The adjustable IOLs may be fabricated with any suitable method that results in a first polymer matrix with one or more components which comprise the Macromer dispersed therein, and where the Macromer is capable of stimulus-induced polymerization to form a second polymer matrix. In general, the method for making an inventive IOL is the same as that for making an inventive optical element. In one embodiment, the method comprises:
- mixing a first polymer matrix composition with a Macromer to form a reaction mixture;
- placing the reaction mixture into a mold;
- polymerizing the first polymer matrix composition to form said optical element; and
- removing the optical element from the mold.
- The type of mold that is used will depend on the optical element being made. For example, if the optical element is a prism, then a mold in the shape of a prism is used. Similarly, if the optical element is an intraoccular lens, then an intraoccular lens mold is used and so forth. As described previously, the first polymer matrix composition comprises one or more monomers for forming the first polymer matrix and optionally includes any number of formulation auxiliaries that either modulate the polymerization reaction or improve any property (whether or not related to the optical characteristic) of the optical element. Similarly, the Macromer comprises one or more components that together are capable of stimulus-induced polymerization to form the second polymer matrix. Because flexible and foldable intraoccular lenses generally permit smaller incisions, it is preferred that both the first polymer matrix composition and the Macromer include one or more silicone-based or low T g acrylic monomers when the inventive method is used to make IOLs.
- Once the adjustable optical element has been formed, it is then implanted in a non-human test subject. Implantation entails removal of the existing lens by phaecoemulsification and extraction of the lens debries. This is followed by implantation of the element using standard surgical procedures.
- After the eye has had sufficient time to heal, (1 to 2 weeks) the eye is then examined for evidence of inflammation. The same time, operability testing can also be conducted.
- Operability testing involves attempting to manipulate the properties of the implant in vivo followed by an evaluation as to whether the desired changes have occurred.
- In the preferred embodiment, this entails exposing at least a portion of the implant to an external stimulus so as to induce a change in the properties of the implant. In one embodiment, ultraviolet light is said to induce photopolymerization of macromers in at least a portion of an adjustable IOL polymerization of the macromers causes change in the shape of the IOL and/or the refractive index of the IOL. The extent of the changes is then evaluated to see if the desired optical properties have been achieved.
- Determination of biocompatibility can be accomplished either in vivo or ex vivo or both. Physical examination of the eye can be used to determine the presence of inflammation and their biocompatibility. In some cases, however, it may be necessary to explant the lens and conduct histopathological studies of the eye tissue to determine biocompatibility.
- The determination of operability requires that at least the adjustment phase be done in vivo followed by examination of the lens in vivo or ex vivo. In vivo examination of the lens can be done using an autoretractometer or a Scheimpflug imaging device to determine change in refraction and/or shape. Alternatively, the lens may be explanted after an ajustment lens has been attempted and the changes in the lens can be determined ex vivo.
- Sterilized, adjustable IOLs were implanted in albino rabbit eyes. After clinically following the eyes for one week, the rabbits were sacrificed. The extracted eyes were evaluated, placed in familiar and studied histopathologically. There was no evidence of corneal toxicity, anterior segment inflammation or other signs of lens toxicity.
- A series of adjustable IOLs were prepared for implantation. The IOLs comprised a silicon based polymer matrix with dimethylsiloxane macromer dispersed therein. The safety and operability of the lenses was evaluated in four rabbits. The rabbits were first anesthetized and the existing lens was removed using phaecoemulsification. The IOLs were then implanted into the rabbits.
- The rabbit eyes were exposed to ultraviolet light for 60 to 120 seconds to induce localized polymerization of macromer in the center of the lens.
- The next day, the rabbits were checked physically to determine if any infection develop or if there was any evidence that the lens was not biocompatible. No evidence of incompatibility or infection was noted.
- The lenses were then examined to determine if the desired changes in optical properties had taken place. This was accomplished by explanting the lenses and then evaluating the change in lens power achieved. In this case the power of the lenses increased an average of 0.72 diopters.
- In this set of experiments, 16 adjustable lens were implanted into albino rabbit eyes. The lenses were adjusted in vivo to diopters of approximately −1.0, −0.5, +1.0 and 2.5 using ultraviolet light. The lenses were then evaluated for biocompatibility and operability by sacrificing the rabbits and explanting the lenses. As shown in FIG. 1, four lenses showed a change in diopters of −1.00,D four had a change in diopter of −0.64D, three had a change in diopter of +0.98D and four had a change of +2.68D.
- Histopathological studies of the eyes showed no inflammation. This indicated good biocompatibility for the lens.
- Although the present invention and its advantages have been described in detail, it should be understood that various changes, substitutions and alterations can be made herein without departing from the spirit and scope of the invention as defined by the appended claims. Moreover, the scope of the present application is not intended to be limited to the particular embodiments of the process, machine, manufacture, composition of matter, means, methods and steps described in the specification. As one of ordinary skill in the art will readily appreciate from the disclosure of the present invention, processes, machines, manufacture, compositions of matter, means, methods, or steps, presently existing or later to be developed that perform substantially the same function or achieve substantially the same result as the corresponding embodiments described herein may be utilized according to the present invention. Accordingly, the appended claims are intended to include within their scope such processes, machines, manufacture, compositions of matter, means, methods, or steps.
Claims (26)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/192,017 US20030048411A1 (en) | 1999-01-12 | 2002-07-10 | Intraoccular lenses capable of in vivo power adjustment and method for same |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11561799P | 1999-01-12 | 1999-01-12 | |
| US13287199P | 1999-05-05 | 1999-05-05 | |
| US14029899P | 1999-06-17 | 1999-06-17 | |
| US09/416,044 US6450642B1 (en) | 1999-01-12 | 1999-10-08 | Lenses capable of post-fabrication power modification |
| US10/192,017 US20030048411A1 (en) | 1999-01-12 | 2002-07-10 | Intraoccular lenses capable of in vivo power adjustment and method for same |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/416,044 Continuation-In-Part US6450642B1 (en) | 1999-01-12 | 1999-10-08 | Lenses capable of post-fabrication power modification |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030048411A1 true US20030048411A1 (en) | 2003-03-13 |
Family
ID=27494026
Family Applications (10)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/416,044 Expired - Lifetime US6450642B1 (en) | 1999-01-12 | 1999-10-08 | Lenses capable of post-fabrication power modification |
| US09/991,560 Abandoned US20020167735A1 (en) | 1999-01-12 | 2001-11-21 | Optical elements capable of post-fabrication modulus change |
| US10/177,722 Abandoned US20030090624A1 (en) | 1999-01-12 | 2002-06-18 | Lenses capable of post-fabrication power modification |
| US10/175,552 Expired - Lifetime US7210783B2 (en) | 1999-01-12 | 2002-06-18 | Lenses capable of post-fabrication power modification |
| US10/176,947 Abandoned US20030090013A1 (en) | 1999-01-12 | 2002-06-18 | Lenses capable of post-fabrication power modification |
| US10/192,017 Abandoned US20030048411A1 (en) | 1999-01-12 | 2002-07-10 | Intraoccular lenses capable of in vivo power adjustment and method for same |
| US10/223,086 Expired - Lifetime US6813097B2 (en) | 1999-01-12 | 2002-08-15 | Lenses capable of post-fabrication modulus change |
| US10/358,065 Expired - Lifetime US6824266B2 (en) | 1999-01-12 | 2003-02-03 | Lenses capable of post-fabrication power modification |
| US11/454,472 Expired - Fee Related US7837326B2 (en) | 1999-01-12 | 2006-06-16 | Lenses capable of post-fabrication power modification |
| US11/743,119 Expired - Fee Related US7798644B2 (en) | 1999-01-12 | 2007-05-01 | Lenses capable of post-fabrication power modification |
Family Applications Before (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/416,044 Expired - Lifetime US6450642B1 (en) | 1999-01-12 | 1999-10-08 | Lenses capable of post-fabrication power modification |
| US09/991,560 Abandoned US20020167735A1 (en) | 1999-01-12 | 2001-11-21 | Optical elements capable of post-fabrication modulus change |
| US10/177,722 Abandoned US20030090624A1 (en) | 1999-01-12 | 2002-06-18 | Lenses capable of post-fabrication power modification |
| US10/175,552 Expired - Lifetime US7210783B2 (en) | 1999-01-12 | 2002-06-18 | Lenses capable of post-fabrication power modification |
| US10/176,947 Abandoned US20030090013A1 (en) | 1999-01-12 | 2002-06-18 | Lenses capable of post-fabrication power modification |
Family Applications After (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/223,086 Expired - Lifetime US6813097B2 (en) | 1999-01-12 | 2002-08-15 | Lenses capable of post-fabrication modulus change |
| US10/358,065 Expired - Lifetime US6824266B2 (en) | 1999-01-12 | 2003-02-03 | Lenses capable of post-fabrication power modification |
| US11/454,472 Expired - Fee Related US7837326B2 (en) | 1999-01-12 | 2006-06-16 | Lenses capable of post-fabrication power modification |
| US11/743,119 Expired - Fee Related US7798644B2 (en) | 1999-01-12 | 2007-05-01 | Lenses capable of post-fabrication power modification |
Country Status (12)
| Country | Link |
|---|---|
| US (10) | US6450642B1 (en) |
| EP (1) | EP1139921B1 (en) |
| JP (2) | JP2004500585A (en) |
| CN (1) | CN1306918C (en) |
| AT (1) | ATE355800T1 (en) |
| AU (1) | AU766157B2 (en) |
| BR (1) | BR9916895A (en) |
| CA (1) | CA2360583A1 (en) |
| DE (1) | DE69935449T2 (en) |
| IL (1) | IL144245A0 (en) |
| MX (1) | MXPA01007051A (en) |
| WO (1) | WO2000041650A1 (en) |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040049174A1 (en) * | 2000-03-21 | 2004-03-11 | Peyman Gholam A. | Adjustable inlay with multizone polymerization |
| US20050113911A1 (en) * | 2002-10-17 | 2005-05-26 | Peyman Gholam A. | Adjustable intraocular lens for insertion into the capsular bag |
| US6905641B2 (en) | 2000-09-26 | 2005-06-14 | Calhoun Vision, Inc. | Delivery system for post-operative power adjustment of adjustable lens |
| US20050182489A1 (en) * | 2001-04-27 | 2005-08-18 | Peyman Gholam A. | Intraocular lens adapted for adjustment via laser after implantation |
| US20060084949A1 (en) * | 2000-03-21 | 2006-04-20 | Peyman Gholam A | Method and apparatus for accommodating intraocular lens |
| US20060216329A1 (en) * | 2000-03-21 | 2006-09-28 | Peyman Gholam A | Drug delivery system and method |
| US20070100443A1 (en) * | 2005-10-27 | 2007-05-03 | Peyman Gholam A | Intraocular lens adapted for accommodation via electrical signals |
| US20110255255A1 (en) * | 2004-04-16 | 2011-10-20 | Sensors For Medicine And Science, Inc. | Housing for a circuit that is to be implanted in-vivo and process of making the same |
| US8752958B2 (en) | 1999-03-01 | 2014-06-17 | Boston Innovative Optics, Inc. | System and method for increasing the depth of focus of the human eye |
| US9204962B2 (en) | 2013-03-13 | 2015-12-08 | Acufocus, Inc. | In situ adjustable optical mask |
| US9427922B2 (en) | 2013-03-14 | 2016-08-30 | Acufocus, Inc. | Process for manufacturing an intraocular lens with an embedded mask |
| US9545303B2 (en) | 2011-12-02 | 2017-01-17 | Acufocus, Inc. | Ocular mask having selective spectral transmission |
| US9681800B2 (en) | 2005-10-27 | 2017-06-20 | The Arizona Board Of Regents On Behalf Of The University Of Arizona | Holographic adaptive see-through phoropter |
| US11266495B2 (en) | 2019-10-20 | 2022-03-08 | Rxsight, Inc. | Light adjustable intraocular lens with a modulable absorption front protection layer |
Families Citing this family (237)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030151831A1 (en) * | 2001-12-28 | 2003-08-14 | Sandstedt Christian A. | Light adjustable multifocal lenses |
| US7281795B2 (en) * | 1999-01-12 | 2007-10-16 | Calhoun Vision, Inc. | Light adjustable multifocal lenses |
| US20050099597A1 (en) * | 2002-12-24 | 2005-05-12 | Calhoun Vision | Light adjustable multifocal lenses |
| US20030128336A1 (en) * | 2001-12-28 | 2003-07-10 | Jethmalani Jagdish M. | Customized lenses |
| US6450642B1 (en) * | 1999-01-12 | 2002-09-17 | California Institute Of Technology | Lenses capable of post-fabrication power modification |
| US20060238702A1 (en) | 1999-04-30 | 2006-10-26 | Advanced Medical Optics, Inc. | Ophthalmic lens combinations |
| DE19932902A1 (en) | 1999-07-12 | 2001-01-25 | Beiersdorf Ag | Data storage |
| DE19935775A1 (en) * | 1999-07-26 | 2001-02-08 | Beiersdorf Ag | Data storage and method for writing information to a data storage |
| DE10008328A1 (en) * | 2000-02-23 | 2002-01-31 | Tesa Ag | Data memory used for storing data has a lacquer layer arranged as an adhesion layer between neighboring polymer film layers |
| EP1658829A1 (en) * | 2000-03-20 | 2006-05-24 | California Institute of Technology | Application of wavefront sensor to lenses capable of post-fabrication power modification |
| JP3677240B2 (en) * | 2000-03-20 | 2005-07-27 | カリフォルニア インスティテュート オブ テクノロジー | Application of wavefront sensor to lens with adjustable magnification after manufacturing |
| AU2001259755A1 (en) * | 2000-05-10 | 2001-11-20 | California Institute Of Technology | Phase contrast variation of a photo-induced refractive material |
| DE10028113A1 (en) * | 2000-06-07 | 2001-12-20 | Beiersdorf Ag | Data memory used in a running gear comprises an optically readable and writable information carrier having a polymer film, and an absorber assigned to the polymer film |
| US6626538B1 (en) * | 2000-07-12 | 2003-09-30 | Peter N. Arrowsmith | Method for determining the power of an intraocular lens used for the treatment of myopia |
| DE10039372C2 (en) | 2000-08-11 | 2003-05-15 | Tesa Scribos Gmbh | Holographic data storage |
| DE10039370A1 (en) * | 2000-08-11 | 2002-02-28 | Eml Europ Media Lab Gmbh | Holographic data storage |
| DE10039374A1 (en) * | 2000-08-11 | 2002-02-21 | Eml Europ Media Lab Gmbh | Holographic data storage |
| AU2001294818B2 (en) * | 2000-09-26 | 2006-08-17 | Calhoun Vision, Inc. | Power adjustment of adjustable lens |
| CA2423225A1 (en) | 2000-10-11 | 2002-04-18 | Ben C. Platt | Light adjustable aberration conjugator |
| US7293871B2 (en) | 2000-11-27 | 2007-11-13 | Ophthonix, Inc. | Apparatus and method of correcting higher-order aberrations of the human eye |
| US6813082B2 (en) * | 2000-11-27 | 2004-11-02 | Ophthonix, Inc. | Wavefront aberrator and method of manufacturing |
| DE10060235A1 (en) * | 2000-12-05 | 2002-06-13 | Tesa Ag | Use of a packing tape as a holographic data carrier |
| US6884261B2 (en) | 2001-01-25 | 2005-04-26 | Visiogen, Inc. | Method of preparing an intraocular lens for implantation |
| US7780729B2 (en) | 2004-04-16 | 2010-08-24 | Visiogen, Inc. | Intraocular lens |
| US8062361B2 (en) | 2001-01-25 | 2011-11-22 | Visiogen, Inc. | Accommodating intraocular lens system with aberration-enhanced performance |
| US6786934B2 (en) * | 2001-01-25 | 2004-09-07 | Visiogen, Inc. | Biasing element for intraocular lens system |
| US20030078657A1 (en) | 2001-01-25 | 2003-04-24 | Gholam-Reza Zadno-Azizi | Materials for use in accommodating intraocular lens system |
| US20030078658A1 (en) | 2001-01-25 | 2003-04-24 | Gholam-Reza Zadno-Azizi | Single-piece accomodating intraocular lens system |
| WO2002076338A2 (en) * | 2001-03-21 | 2002-10-03 | Calhoun Vision | Composition and method for producing shapable implants in vivo and implants produced thereby |
| US7217375B2 (en) | 2001-06-04 | 2007-05-15 | Ophthonix, Inc. | Apparatus and method of fabricating a compensating element for wavefront correction using spatially localized curing of resin mixtures |
| US6855164B2 (en) * | 2001-06-11 | 2005-02-15 | Vision Solutions Technologies, Llc | Multi-focal intraocular lens, and methods for making and using same |
| US7229475B2 (en) | 2001-06-11 | 2007-06-12 | Vision Solutions Technologies, Inc. | Multi-focal intraocular lens, and methods for making and using same |
| US20050119739A1 (en) * | 2001-06-11 | 2005-06-02 | Vision Solution Technologies, Llc | Multi-focal intraocular lens, and methods for making and using same |
| US20030033010A1 (en) * | 2001-06-13 | 2003-02-13 | Hicks Celia R. | Method of improved keratoprosthesis |
| DE10128901A1 (en) * | 2001-06-15 | 2002-12-19 | Tesa Ag | A process for giving information to an optically writable and readable data store with a polymer film for information storage and an absorbing colorant useful for providing information to a data storage device |
| DE10128902A1 (en) * | 2001-06-15 | 2003-10-16 | Tesa Scribos Gmbh | Holographic data storage |
| US7044604B1 (en) | 2001-07-11 | 2006-05-16 | Arrowsmith Peter N | Method for determining the power of an intraocular lens used for the treatment of myopia |
| WO2003011194A1 (en) * | 2001-07-27 | 2003-02-13 | California Institute Of Technology | Intraoccular lenses with power adjustability in vivo |
| US20030060878A1 (en) | 2001-08-31 | 2003-03-27 | Shadduck John H. | Intraocular lens system and method for power adjustment |
| US7434931B2 (en) | 2001-10-25 | 2008-10-14 | Ophthonix | Custom eyeglass manufacturing method |
| US6712466B2 (en) | 2001-10-25 | 2004-03-30 | Ophthonix, Inc. | Eyeglass manufacturing method using variable index layer |
| US6682195B2 (en) | 2001-10-25 | 2004-01-27 | Ophthonix, Inc. | Custom eyeglass manufacturing method |
| US7241009B2 (en) * | 2001-11-21 | 2007-07-10 | Calhoun Vision, Inc. | Crosslinking of silicones presence of functionalized silicones |
| US7237893B2 (en) * | 2001-12-28 | 2007-07-03 | Chang Shiao H | Light adjustable lenses capable of post-fabrication power modification via multi-photon processes |
| US20030151825A1 (en) * | 2001-12-28 | 2003-08-14 | California Institute Of Technology | Polyacrylate-based light adjustable optical element |
| WO2003057023A1 (en) | 2002-01-10 | 2003-07-17 | Carl Zeiss Meditec Ag | Arrangement and method for illuminating the lens of the human eye |
| US7763069B2 (en) | 2002-01-14 | 2010-07-27 | Abbott Medical Optics Inc. | Accommodating intraocular lens with outer support structure |
| US7115305B2 (en) | 2002-02-01 | 2006-10-03 | California Institute Of Technology | Method of producing regular arrays of nano-scale objects using nano-structured block-copolymeric materials |
| US8048155B2 (en) | 2002-02-02 | 2011-11-01 | Powervision, Inc. | Intraocular implant devices |
| US6935743B2 (en) | 2002-02-06 | 2005-08-30 | John H. Shadduck | Adaptive optic lens and method of making |
| US6836371B2 (en) | 2002-07-11 | 2004-12-28 | Ophthonix, Inc. | Optical elements and methods for making thereof |
| US7420743B2 (en) | 2002-07-11 | 2008-09-02 | Ophthonix, Inc. | Optical elements and methods for making thereof |
| US6966649B2 (en) * | 2002-08-12 | 2005-11-22 | John H Shadduck | Adaptive optic lens system and method of use |
| US7662180B2 (en) | 2002-12-05 | 2010-02-16 | Abbott Medical Optics Inc. | Accommodating intraocular lens and method of manufacture thereof |
| CA2508143A1 (en) | 2002-12-12 | 2004-06-24 | Powervision, Inc. | Lens system for power adjustment using micropumps |
| US8328869B2 (en) | 2002-12-12 | 2012-12-11 | Powervision, Inc. | Accommodating intraocular lenses and methods of use |
| EP2559405A3 (en) * | 2002-12-12 | 2013-06-26 | PowerVision, Inc. | Accommodating intraocular lens system |
| US7637947B2 (en) | 2002-12-12 | 2009-12-29 | Powervision, Inc. | Accommodating intraocular lens system having spherical aberration compensation and method |
| US10835373B2 (en) | 2002-12-12 | 2020-11-17 | Alcon Inc. | Accommodating intraocular lenses and methods of use |
| US8361145B2 (en) | 2002-12-12 | 2013-01-29 | Powervision, Inc. | Accommodating intraocular lens system having circumferential haptic support and method |
| US7217288B2 (en) | 2002-12-12 | 2007-05-15 | Powervision, Inc. | Accommodating intraocular lens having peripherally actuated deflectable surface and method |
| US7615056B2 (en) | 2003-02-14 | 2009-11-10 | Visiogen, Inc. | Method and device for compacting an intraocular lens |
| DE10307741A1 (en) | 2003-02-24 | 2004-09-02 | Carl Zeiss Meditec Ag | Arrangement for improving the image field in ophthalmic devices |
| DE10314944A1 (en) | 2003-04-02 | 2004-10-14 | Carl Zeiss Meditec Ag | Illumination and radiation unit for ophthalmic devices |
| AU2004292160B2 (en) * | 2003-11-14 | 2010-12-23 | Ophthonix, Inc. | Eyeglass manufacturing method |
| US7188950B2 (en) * | 2003-11-14 | 2007-03-13 | Ophthonix, Inc. | Eyeglass dispensing method |
| US20050131535A1 (en) | 2003-12-15 | 2005-06-16 | Randall Woods | Intraocular lens implant having posterior bendable optic |
| JP3765425B2 (en) * | 2004-01-26 | 2006-04-12 | 日立工機株式会社 | Portable electric cutting machine |
| US7645300B2 (en) | 2004-02-02 | 2010-01-12 | Visiogen, Inc. | Injector for intraocular lens system |
| CA2555306A1 (en) * | 2004-02-06 | 2005-08-18 | Nymox Corporation | Humanized antibody |
| IL161706A0 (en) | 2004-04-29 | 2004-09-27 | Nulens Ltd | Intraocular lens fixation device |
| US9005282B2 (en) * | 2004-04-30 | 2015-04-14 | Calhoun Vision, Inc. | Intraocular lens system with injectable accommodation material |
| WO2005107649A2 (en) * | 2004-04-30 | 2005-11-17 | Calhoun Vision, Inc. | Injectable accommodation composition |
| US9713527B2 (en) * | 2004-04-30 | 2017-07-25 | Rxsight, Inc. | Multilens intraocular lens system with injectable accommodation material |
| WO2006041535A2 (en) | 2004-05-05 | 2006-04-20 | California Institute Of Technology | Capillary lithography of nanofiber arrays |
| US20050260388A1 (en) * | 2004-05-21 | 2005-11-24 | Lai Shui T | Apparatus and method of fabricating an ophthalmic lens for wavefront correction using spatially localized curing of photo-polymerization materials |
| DE602005018221D1 (en) * | 2004-07-30 | 2010-01-21 | Novartis Ag | METHOD FOR THE PRODUCTION OF OPHTHALMIC LENSES WITH MODULATED ENERGY |
| US20060027939A1 (en) * | 2004-08-03 | 2006-02-09 | Axel Brait | Method for making novel compositions capable of post fabrication modification |
| US20060043623A1 (en) * | 2004-08-27 | 2006-03-02 | Powell P M | Masked precure of ophthalmic lenses: systems and methods thereof |
| US20060050228A1 (en) * | 2004-09-07 | 2006-03-09 | Lai Shui T | Method for stabilizing refractive index profiles using polymer mixtures |
| US8000013B2 (en) * | 2004-09-07 | 2011-08-16 | Ophthonix, Inc. | Tinted lenses that correct for high order aberrations |
| US7371804B2 (en) * | 2004-09-07 | 2008-05-13 | Ophthonix, Inc. | Monomers and polymers for optical elements |
| US9872763B2 (en) * | 2004-10-22 | 2018-01-23 | Powervision, Inc. | Accommodating intraocular lenses |
| US8021967B2 (en) | 2004-11-01 | 2011-09-20 | California Institute Of Technology | Nanoscale wicking methods and devices |
| US8377123B2 (en) | 2004-11-10 | 2013-02-19 | Visiogen, Inc. | Method of implanting an intraocular lens |
| US20060173539A1 (en) | 2005-01-31 | 2006-08-03 | Yichieh Shiuey | Corneal implants and methods and systems for placement |
| US9999497B2 (en) * | 2005-01-31 | 2018-06-19 | Yichieh Shiuey | Corneal implants and methods and systems for placement |
| US8029515B2 (en) | 2005-01-31 | 2011-10-04 | Yichieh Shiuey | Corneal implants and methods and systems for placement |
| CN101203192B (en) | 2005-03-30 | 2010-09-15 | 纽镜有限公司 | Adjustable intraocular lens assemblies and discrete components thereof |
| US7781100B2 (en) * | 2005-05-10 | 2010-08-24 | Advanced Lithium Electrochemistry Co., Ltd | Cathode material for manufacturing rechargeable battery |
| BRPI0611968B8 (en) * | 2005-06-13 | 2021-07-27 | Alcon Inc | ophthalmic and otolaryngological devices, their material, and intraocular lens |
| US8579970B1 (en) | 2005-06-27 | 2013-11-12 | Visiogen, Inc. | Magnifying intraocular lens |
| DE102005032041A1 (en) | 2005-07-08 | 2007-01-18 | Carl Zeiss Meditec Ag | Device and method for changing an optical and / or mechanical property of a lens implanted in an eye |
| US9636213B2 (en) | 2005-09-30 | 2017-05-02 | Abbott Medical Optics Inc. | Deformable intraocular lenses and lens systems |
| EP1933751A4 (en) | 2005-10-13 | 2009-12-02 | Shui T Lai | Intrastromal refractive surgery by inducing shape change of the cornea |
| US8657877B2 (en) * | 2005-11-14 | 2014-02-25 | Vision Solutions Technologies, Inc. | Multi-focal prosthesis, and methods for making and using same |
| US7964693B2 (en) * | 2005-12-29 | 2011-06-21 | The University Of Akron | Photocurable polymers for ophthalmic applications |
| US7726811B2 (en) * | 2006-02-14 | 2010-06-01 | Lai Shui T | Subjective wavefront refraction using continuously adjustable wave plates of Zernike function |
| US7701641B2 (en) * | 2006-03-20 | 2010-04-20 | Ophthonix, Inc. | Materials and methods for producing lenses |
| WO2007147152A2 (en) * | 2006-06-15 | 2007-12-21 | Lai Shui T | High visual acuity contact lenses |
| US8071693B2 (en) * | 2006-06-22 | 2011-12-06 | Sabic Innovative Plastics Ip B.V. | Polysiloxane/polyimide copolymers and blends thereof |
| US7789910B2 (en) | 2006-06-28 | 2010-09-07 | Bausch & Lomb Incorporated | Optical material and method for modifying the refractive index |
| US20080001320A1 (en) * | 2006-06-28 | 2008-01-03 | Knox Wayne H | Optical Material and Method for Modifying the Refractive Index |
| TWI399228B (en) | 2006-07-21 | 2013-06-21 | Alcon Inc | Low-tack ophthalmic and otorhinolaryngological device materials |
| US7959284B2 (en) * | 2006-07-25 | 2011-06-14 | Lai Shui T | Method of making high precision optics having a wavefront profile |
| US20080027537A1 (en) * | 2006-07-26 | 2008-01-31 | Calhoun Vision, Inc. | Method for improved retinal safety using the light adjustable lens (LAL) |
| US8622544B2 (en) * | 2006-08-31 | 2014-01-07 | Nike, Inc. | Adjustable spectral transmittance curved lens eyewear |
| US7828434B2 (en) * | 2006-08-31 | 2010-11-09 | Nike, Inc. | Zone switched sports training eyewear |
| US8708484B2 (en) | 2006-08-31 | 2014-04-29 | Nike, Inc. | Adjustable spectral transmittance eyewear |
| WO2008036695A2 (en) * | 2006-09-18 | 2008-03-27 | Lai Shui T | Customized contact lenses for reducing aberrations of the eye |
| US20080103592A1 (en) * | 2006-10-30 | 2008-05-01 | Calhoun Vision, Inc. | Piggyback lenses |
| US8403984B2 (en) | 2006-11-29 | 2013-03-26 | Visiogen, Inc. | Apparatus and methods for compacting an intraocular lens |
| AU2007338100B2 (en) | 2006-12-22 | 2014-01-30 | Amo Groningen Bv | Accommodating intraocular lens, lens system and frame therefor |
| US20080161914A1 (en) | 2006-12-29 | 2008-07-03 | Advanced Medical Optics, Inc. | Pre-stressed haptic for accommodating intraocular lens |
| EP2112932B1 (en) | 2007-02-21 | 2014-12-17 | PowerVision, Inc. | Polymeric materials suitable for ophthalmic devices and methods of manufacture |
| US20080236864A1 (en) * | 2007-03-28 | 2008-10-02 | General Electric Company | Cross linked polysiloxane/polyimide copolymers, methods of making, blends thereof, and articles derived therefrom |
| CN101754728B (en) | 2007-07-23 | 2013-09-18 | 力景公司 | Lens delivery system |
| US8314927B2 (en) | 2007-07-23 | 2012-11-20 | Powervision, Inc. | Systems and methods for testing intraocular lenses |
| US8968396B2 (en) | 2007-07-23 | 2015-03-03 | Powervision, Inc. | Intraocular lens delivery systems and methods of use |
| EP2178463B1 (en) | 2007-07-23 | 2013-09-04 | PowerVision, Inc. | Accommodating intraocular lenses |
| AU2008279167B2 (en) | 2007-07-23 | 2014-10-09 | Alcon Inc. | Post-implant lens power modification |
| US20090118828A1 (en) * | 2007-11-06 | 2009-05-07 | Altmann Griffith E | Light-adjustable multi-element ophthalmic lens |
| WO2009070438A1 (en) | 2007-11-30 | 2009-06-04 | Bausch & Lomb Incorporated | Optical material and method for modifying the refractive index |
| US8425595B2 (en) | 2008-03-12 | 2013-04-23 | Visiogen, Inc. | Method for inserting an intraocular lens |
| US8034108B2 (en) | 2008-03-28 | 2011-10-11 | Abbott Medical Optics Inc. | Intraocular lens having a haptic that includes a cap |
| US9232993B2 (en) | 2008-04-04 | 2016-01-12 | Battelle Memorial Institute | Adjustable intraocular lens |
| WO2015038614A1 (en) | 2013-09-12 | 2015-03-19 | Battelle Memorial Institute | Methods of altering the refractive index of materials |
| US10254562B2 (en) * | 2008-04-04 | 2019-04-09 | Battelle Memorial Institute | Methods for tailoring the refractive index of lenses |
| US10018853B2 (en) | 2008-04-04 | 2018-07-10 | Battelle Memorial Institute | Methods of altering the refractive index of materials |
| US9060847B2 (en) * | 2008-05-19 | 2015-06-23 | University Of Rochester | Optical hydrogel material with photosensitizer and method for modifying the refractive index |
| US10299913B2 (en) | 2009-01-09 | 2019-05-28 | Powervision, Inc. | Accommodating intraocular lenses and methods of use |
| US8222360B2 (en) | 2009-02-13 | 2012-07-17 | Visiogen, Inc. | Copolymers for intraocular lens systems |
| CA2754775C (en) | 2009-03-04 | 2016-09-27 | Aaren Scientific Inc. | System for characterizing a cornea and obtaining an ophthalmic lens |
| US8292952B2 (en) | 2009-03-04 | 2012-10-23 | Aaren Scientific Inc. | System for forming and modifying lenses and lenses formed thereby |
| US8646916B2 (en) | 2009-03-04 | 2014-02-11 | Perfect Ip, Llc | System for characterizing a cornea and obtaining an opthalmic lens |
| EP2243622A3 (en) * | 2009-04-22 | 2015-06-03 | Canon Kabushiki Kaisha | Method for producing optical part |
| EP2445446B1 (en) | 2009-06-26 | 2019-01-09 | Johnson & Johnson Surgical Vision, Inc. | Accommodating intraocular lenses |
| CA2770074C (en) | 2009-08-03 | 2017-09-05 | Abbott Medical Optics Inc. | Intraocular lens for providing accomodative vision |
| JP5894076B2 (en) | 2009-08-31 | 2016-03-23 | パワーヴィジョン・インコーポレーテッド | Lens capsule size estimation method |
| EP2317511B1 (en) * | 2009-11-03 | 2012-03-07 | Bayer MaterialScience AG | Photopolymer formulations with adjustable mechanical module Guv |
| US8900298B2 (en) | 2010-02-23 | 2014-12-02 | Powervision, Inc. | Fluid for accommodating intraocular lenses |
| ES2634491T3 (en) | 2010-07-07 | 2017-09-28 | California Institute Of Technology | Photoinitiated on demand polymerization |
| WO2012006616A2 (en) | 2010-07-09 | 2012-01-12 | Powervision, Inc. | Intraocular lens delivery devices and methods of use |
| US20150257873A1 (en) | 2010-09-30 | 2015-09-17 | Yichieh Shiuey | Reversibly deformable artificial cornea and methods for implantation |
| EP2670347B1 (en) | 2011-02-04 | 2020-04-08 | ForSight Vision6, Inc. | Intraocular accommodating lens |
| EP3928744A1 (en) | 2011-03-24 | 2021-12-29 | Alcon Inc. | Intraocular lens loading systems and methods of use |
| US9144491B2 (en) | 2011-06-02 | 2015-09-29 | University Of Rochester | Method for modifying the refractive index of an optical material |
| US10307292B2 (en) | 2011-07-18 | 2019-06-04 | Mor Research Applications Ltd | Device for adjusting the intraocular pressure |
| US11191637B2 (en) | 2011-09-16 | 2021-12-07 | Rxsight, Inc. | Blended extended depth of focus light adjustable lens with laterally offset axes |
| US11135052B2 (en) | 2011-09-16 | 2021-10-05 | Rxsight, Inc. | Method of adjusting a blended extended depth of focus light adjustable lens with laterally offset axes |
| US10874505B2 (en) | 2011-09-16 | 2020-12-29 | Rxsight, Inc. | Using the light adjustable lens (LAL) to increase the depth of focus by inducing targeted amounts of asphericity |
| US10433949B2 (en) | 2011-11-08 | 2019-10-08 | Powervision, Inc. | Accommodating intraocular lenses |
| US8900300B1 (en) | 2012-02-22 | 2014-12-02 | Omega Ophthalmics Llc | Prosthetic capsular bag and method of inserting the same |
| US9084674B2 (en) | 2012-05-02 | 2015-07-21 | Abbott Medical Optics Inc. | Intraocular lens with shape changing capability to provide enhanced accomodation and visual acuity |
| US8798332B2 (en) | 2012-05-15 | 2014-08-05 | Google Inc. | Contact lenses |
| US9158133B1 (en) | 2012-07-26 | 2015-10-13 | Google Inc. | Contact lens employing optical signals for power and/or communication |
| US9298020B1 (en) | 2012-07-26 | 2016-03-29 | Verily Life Sciences Llc | Input system |
| US9523865B2 (en) | 2012-07-26 | 2016-12-20 | Verily Life Sciences Llc | Contact lenses with hybrid power sources |
| US8857981B2 (en) | 2012-07-26 | 2014-10-14 | Google Inc. | Facilitation of contact lenses with capacitive sensors |
| US8919953B1 (en) | 2012-08-02 | 2014-12-30 | Google Inc. | Actuatable contact lenses |
| US8971978B2 (en) | 2012-08-21 | 2015-03-03 | Google Inc. | Contact lens with integrated pulse oximeter |
| US9696564B1 (en) | 2012-08-21 | 2017-07-04 | Verily Life Sciences Llc | Contact lens with metal portion and polymer layer having indentations |
| US9111473B1 (en) | 2012-08-24 | 2015-08-18 | Google Inc. | Input system |
| US8820934B1 (en) | 2012-09-05 | 2014-09-02 | Google Inc. | Passive surface acoustic wave communication |
| US20140192315A1 (en) | 2012-09-07 | 2014-07-10 | Google Inc. | In-situ tear sample collection and testing using a contact lens |
| US9398868B1 (en) | 2012-09-11 | 2016-07-26 | Verily Life Sciences Llc | Cancellation of a baseline current signal via current subtraction within a linear relaxation oscillator-based current-to-frequency converter circuit |
| US10010270B2 (en) | 2012-09-17 | 2018-07-03 | Verily Life Sciences Llc | Sensing system |
| US9326710B1 (en) | 2012-09-20 | 2016-05-03 | Verily Life Sciences Llc | Contact lenses having sensors with adjustable sensitivity |
| US8960898B1 (en) | 2012-09-24 | 2015-02-24 | Google Inc. | Contact lens that restricts incoming light to the eye |
| US8870370B1 (en) | 2012-09-24 | 2014-10-28 | Google Inc. | Contact lens that facilitates antenna communication via sensor impedance modulation |
| US8979271B2 (en) | 2012-09-25 | 2015-03-17 | Google Inc. | Facilitation of temperature compensation for contact lens sensors and temperature sensing |
| US20140088372A1 (en) | 2012-09-25 | 2014-03-27 | Google Inc. | Information processing method |
| US8989834B2 (en) | 2012-09-25 | 2015-03-24 | Google Inc. | Wearable device |
| US8960899B2 (en) | 2012-09-26 | 2015-02-24 | Google Inc. | Assembling thin silicon chips on a contact lens |
| US8821811B2 (en) | 2012-09-26 | 2014-09-02 | Google Inc. | In-vitro contact lens testing |
| US9884180B1 (en) | 2012-09-26 | 2018-02-06 | Verily Life Sciences Llc | Power transducer for a retinal implant using a contact lens |
| US8985763B1 (en) | 2012-09-26 | 2015-03-24 | Google Inc. | Contact lens having an uneven embedded substrate and method of manufacture |
| ES2457840B1 (en) | 2012-09-28 | 2015-02-16 | Universidad De Murcia | Variable power accommodative intraocular lens and variable power accommodative intraocular lens set and capsular ring |
| US9063351B1 (en) | 2012-09-28 | 2015-06-23 | Google Inc. | Input detection system |
| US8965478B2 (en) | 2012-10-12 | 2015-02-24 | Google Inc. | Microelectrodes in an ophthalmic electrochemical sensor |
| US9176332B1 (en) | 2012-10-24 | 2015-11-03 | Google Inc. | Contact lens and method of manufacture to improve sensor sensitivity |
| US9757056B1 (en) | 2012-10-26 | 2017-09-12 | Verily Life Sciences Llc | Over-molding of sensor apparatus in eye-mountable device |
| US8998984B2 (en) | 2013-01-14 | 2015-04-07 | Andrew F. Phillips | Adjustable toric intraocular lens |
| US8874182B2 (en) | 2013-01-15 | 2014-10-28 | Google Inc. | Encapsulated electronics |
| US9289954B2 (en) | 2013-01-17 | 2016-03-22 | Verily Life Sciences Llc | Method of ring-shaped structure placement in an eye-mountable device |
| US20140209481A1 (en) | 2013-01-25 | 2014-07-31 | Google Inc. | Standby Biasing Of Electrochemical Sensor To Reduce Sensor Stabilization Time During Measurement |
| US9636016B1 (en) | 2013-01-25 | 2017-05-02 | Verily Life Sciences Llc | Eye-mountable devices and methods for accurately placing a flexible ring containing electronics in eye-mountable devices |
| US9608433B2 (en) | 2013-03-14 | 2017-03-28 | Hubbell Incorporated | GFCI test monitor circuit |
| WO2014145562A1 (en) | 2013-03-15 | 2014-09-18 | Powervision, Inc. | Intraocular lens storage and loading devices and methods of use |
| ES2561756T3 (en) * | 2013-03-18 | 2016-02-29 | Polight As | Deformable polymeric lens |
| US9161712B2 (en) | 2013-03-26 | 2015-10-20 | Google Inc. | Systems and methods for encapsulating electronics in a mountable device |
| US9113829B2 (en) | 2013-03-27 | 2015-08-25 | Google Inc. | Systems and methods for encapsulating electronics in a mountable device |
| US20140371560A1 (en) | 2013-06-14 | 2014-12-18 | Google Inc. | Body-Mountable Devices and Methods for Embedding a Structure in a Body-Mountable Device |
| US9084561B2 (en) | 2013-06-17 | 2015-07-21 | Google Inc. | Symmetrically arranged sensor electrodes in an ophthalmic electrochemical sensor |
| US9948895B1 (en) | 2013-06-18 | 2018-04-17 | Verily Life Sciences Llc | Fully integrated pinhole camera for eye-mountable imaging system |
| US9685689B1 (en) | 2013-06-27 | 2017-06-20 | Verily Life Sciences Llc | Fabrication methods for bio-compatible devices |
| US9814387B2 (en) | 2013-06-28 | 2017-11-14 | Verily Life Sciences, LLC | Device identification |
| US9028772B2 (en) | 2013-06-28 | 2015-05-12 | Google Inc. | Methods for forming a channel through a polymer layer using one or more photoresist layers |
| US9492118B1 (en) | 2013-06-28 | 2016-11-15 | Life Sciences Llc | Pre-treatment process for electrochemical amperometric sensor |
| US9307901B1 (en) | 2013-06-28 | 2016-04-12 | Verily Life Sciences Llc | Methods for leaving a channel in a polymer layer using a cross-linked polymer plug |
| US9572522B2 (en) | 2013-12-20 | 2017-02-21 | Verily Life Sciences Llc | Tear fluid conductivity sensor |
| US9654674B1 (en) | 2013-12-20 | 2017-05-16 | Verily Life Sciences Llc | Image sensor with a plurality of light channels |
| US9366570B1 (en) | 2014-03-10 | 2016-06-14 | Verily Life Sciences Llc | Photodiode operable in photoconductive mode and photovoltaic mode |
| US9184698B1 (en) | 2014-03-11 | 2015-11-10 | Google Inc. | Reference frequency from ambient light signal |
| US9789655B1 (en) | 2014-03-14 | 2017-10-17 | Verily Life Sciences Llc | Methods for mold release of body-mountable devices including microelectronics |
| KR101802574B1 (en) * | 2014-03-28 | 2017-12-01 | 삼성에스디아이 주식회사 | Composition for encapsulating organic light emitting diode device and organic light emitting diode display using prepared the same |
| WO2015148673A1 (en) | 2014-03-28 | 2015-10-01 | Forsight Labs, Llc | Accommodating intraocular lens |
| EP2924085B1 (en) * | 2014-03-28 | 2019-05-08 | Samsung SDI Co., Ltd. | Composition for encapsulation of organic light emitting diode and organic light emitting diode display manufactured using the same |
| KR101861893B1 (en) * | 2014-04-23 | 2018-05-29 | 삼성에스디아이 주식회사 | Composition for encapsulating organic light emitting diode device and organic light emitting diode display using prepared the same |
| WO2015195825A1 (en) | 2014-06-19 | 2015-12-23 | Omega Ophthalmics Llc | Prostheticcapsular devices, systems, and methods |
| US20140358226A1 (en) * | 2014-08-15 | 2014-12-04 | Allen Louis Cohen | Light Adjustable IOL With Diffraction Multifocal |
| US10351675B2 (en) | 2014-12-22 | 2019-07-16 | Adventus Technology, Inc. | Compositions and methods for injectable composition for an accommodating instraocular lens |
| US9358103B1 (en) | 2015-02-10 | 2016-06-07 | Omega Ophthalmics Llc | Prosthetic capsular devices, systems, and methods |
| DE102015102298A1 (en) * | 2015-02-18 | 2016-08-18 | Carl Zeiss Meditec Ag | Ophthalmic composition having at least one molecular switch compound |
| CN107077009B (en) | 2015-05-20 | 2019-11-15 | Rx视觉股份有限公司 | Method for modifying the adjustable lenticular refractive power of light |
| GB2540144A (en) * | 2015-07-06 | 2017-01-11 | Rayner Intraocular Lenses Ltd | Intraocular lens |
| CN111407464B (en) | 2015-11-06 | 2022-12-13 | 爱尔康公司 | Accommodating intraocular lens and method of manufacture |
| EP3389560A1 (en) | 2015-12-15 | 2018-10-24 | The University of Rochester | Refractive corrector incorporating a continuous central phase zone and peripheral phase discontinuities |
| ES2631354B1 (en) | 2016-02-29 | 2019-10-09 | Univ Murcia | INTRAOCULAR OPENING CORRECTING LENS |
| AU2017277989B2 (en) | 2016-06-06 | 2019-11-21 | Omega Ophthalmics Llc | Prosthetic capsular devices, systems, and methods |
| CA3040281C (en) | 2016-10-21 | 2021-04-13 | Omega Ophthalmics Llc | Prosthetic capsular devices |
| EA037705B1 (en) | 2016-10-28 | 2021-05-12 | Форсайт Вижн6, Инк. | Accommodating intraocular lens and system for implantation in an eye |
| US10433951B2 (en) | 2017-05-22 | 2019-10-08 | Rxsight, Inc. | Depth of focus and visual acuity using colorized apodization of intra-ocular lenses |
| US20210322152A1 (en) * | 2020-04-15 | 2021-10-21 | Rxsight, Inc. | Composite light adjustable intraocular lens with adhesion promoter |
| US10966819B2 (en) | 2017-05-29 | 2021-04-06 | Rxsight, Inc. | Composite light adjustable intraocular lens |
| US11707354B2 (en) | 2017-09-11 | 2023-07-25 | Amo Groningen B.V. | Methods and apparatuses to increase intraocular lenses positional stability |
| EP3703611B1 (en) * | 2017-11-01 | 2022-11-23 | Nayam Innovations Pvt Ltd | Photo-responsive shape changing polymer composition for colored optical lens |
| US10456240B2 (en) | 2017-11-24 | 2019-10-29 | Rxsight, Inc. | Patient interface for light adjustable intraocular lens irradiation system |
| US10864075B2 (en) | 2017-12-31 | 2020-12-15 | Rxsight, Inc. | Intraocular lens visualization and tracking system |
| EP3773334A4 (en) | 2018-04-06 | 2021-12-29 | Omega Ophthalmics LLC | Prosthetic capsular devices, systems, and methods |
| US11583389B2 (en) | 2019-04-05 | 2023-02-21 | Amo Groningen B.V. | Systems and methods for correcting photic phenomenon from an intraocular lens and using refractive index writing |
| US11583388B2 (en) | 2019-04-05 | 2023-02-21 | Amo Groningen B.V. | Systems and methods for spectacle independence using refractive index writing with an intraocular lens |
| US11678975B2 (en) | 2019-04-05 | 2023-06-20 | Amo Groningen B.V. | Systems and methods for treating ocular disease with an intraocular lens and refractive index writing |
| US11564839B2 (en) | 2019-04-05 | 2023-01-31 | Amo Groningen B.V. | Systems and methods for vergence matching of an intraocular lens with refractive index writing |
| US11529230B2 (en) | 2019-04-05 | 2022-12-20 | Amo Groningen B.V. | Systems and methods for correcting power of an intraocular lens using refractive index writing |
| KR20220074943A (en) | 2019-10-04 | 2022-06-03 | 알콘 인코포레이티드 | Adjustable Intraocular Lenses and Methods of Postoperative Adjustment of Intraocular Lenses |
| AU2021359888A1 (en) | 2020-10-12 | 2023-06-15 | Omega Ophthalmics Llc | Prosthetic capsular devices, systems, and methods |
Family Cites Families (88)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS525857B2 (en) | 1972-10-23 | 1977-02-17 | ||
| US4008136A (en) | 1974-08-09 | 1977-02-15 | Temple University | Process for the treatment of waste water by heterogeneous photosensitized oxidation |
| JPS5315152A (en) | 1976-07-27 | 1978-02-10 | Canon Inc | Hologram |
| DE2722928C2 (en) | 1977-05-20 | 1983-01-05 | Hans Grohe Gmbh & Co Kg, 7622 Schiltach | Flexible plastic hose |
| US4173475A (en) | 1977-05-31 | 1979-11-06 | Bell Telephone Laboratories, Incorporated | Latent image thick refractive index recordings |
| US4330383A (en) * | 1978-07-18 | 1982-05-18 | Polymer Technology Corporation | Dimensionally stable oxygen permeable hard contact lens material and method of manufacture |
| US4260725A (en) | 1979-12-10 | 1981-04-07 | Bausch & Lomb Incorporated | Hydrophilic contact lens made from polysiloxanes which are thermally bonded to polymerizable groups and which contain hydrophilic sidechains |
| JPS60175009A (en) | 1984-02-21 | 1985-09-09 | Nippon Sheet Glass Co Ltd | Production of plastic optical element having refractive index distribution |
| US4581031A (en) * | 1984-06-22 | 1986-04-08 | Koziol Jeffrey E | Prismatic intraocular lens |
| JPS6127501A (en) | 1984-07-17 | 1986-02-07 | Nippon Sheet Glass Co Ltd | Manufacture of synthetic resin optical element having refractive index distribution |
| US4575373A (en) | 1984-11-02 | 1986-03-11 | Johnson Don R | Laser adjustable intraocular lens and method of altering lens power |
| JPS61190546A (en) * | 1985-02-20 | 1986-08-25 | Central Glass Co Ltd | Resin composition for optical use |
| SE449555B (en) | 1985-02-26 | 1987-05-11 | Birger Pettersson | HOPPABLE CHAIR WITH A VERMEKELLA |
| US4787903A (en) | 1985-07-24 | 1988-11-29 | Grendahl Dennis T | Intraocular lens |
| US4685921A (en) | 1986-02-24 | 1987-08-11 | Peyman Gholam A | Variable refractive power, expandable intraocular lenses |
| US4843136A (en) * | 1986-09-26 | 1989-06-27 | Bayer Aktiengesellschaft | (Meth)-acrylates of siloxanes containing tricyclodecane groups |
| US4790847A (en) | 1987-05-26 | 1988-12-13 | Woods Randall L | Intraocular lens implant having eye focusing capabilities |
| US4846172A (en) | 1987-05-26 | 1989-07-11 | Berlin Michael S | Laser-delivery eye-treatment method |
| US4942112A (en) | 1988-01-15 | 1990-07-17 | E. I. Du Pont De Nemours And Company | Photopolymerizable compositions and elements for refractive index imaging |
| US4816031A (en) | 1988-01-29 | 1989-03-28 | Pfoff David S | Intraocular lens system |
| US4921589A (en) | 1988-12-20 | 1990-05-01 | Allied-Signal Inc. | Polysiloxane bound photosensitizer for producing singlet oxygen |
| JP2508241B2 (en) * | 1989-02-23 | 1996-06-19 | 鹿島建設株式会社 | Safety monitoring device for active seismic control and wind control devices |
| FR2646930B1 (en) * | 1989-05-12 | 1993-04-09 | Essilor Int | PROCESS FOR PRODUCING A DIFFRACTIVE ELEMENT, USABLE IN PARTICULAR IN THE MANUFACTURE OF ARTIFICIAL OPTICAL LENSES, AND LENSES THUS OBTAINED |
| US5112205A (en) * | 1989-05-15 | 1992-05-12 | Sony Corporation | Optical-disk manufacturing apparatus |
| US4944112A (en) | 1989-05-25 | 1990-07-31 | Garmany Douglas L | Lure system with adaptable insitu bill assembly |
| JP2798468B2 (en) | 1990-02-28 | 1998-09-17 | ホーヤ株式会社 | Contact lens material and method of manufacturing contact lens |
| JPH03288102A (en) * | 1990-04-04 | 1991-12-18 | Fujitsu Ltd | Manufacture of light beam shape converting element |
| US5296305A (en) | 1990-05-11 | 1994-03-22 | Esslior International (Compagnie Generale D'optique) | Method of fabricating a lens made of transparent polymer with modulated refracting index |
| FR2661914B1 (en) * | 1990-05-11 | 1994-05-06 | Essilor Internal Cie Gle Optique | METHOD FOR MANUFACTURING A TRANSPARENT POLYMER LENS WITH MODULATED REFRACTION INDEX. |
| EP0472384A3 (en) | 1990-08-16 | 1992-10-28 | Yasuhiro Koike | Plastic optical fiber and its manufacturing method |
| JPH04110110A (en) | 1990-08-30 | 1992-04-10 | Seiko Epson Corp | Molding method of plastic lens provided with prism |
| US5171266A (en) | 1990-09-04 | 1992-12-15 | Wiley Robert G | Variable power intraocular lens with astigmatism correction |
| US5066301A (en) | 1990-10-09 | 1991-11-19 | Wiley Robert G | Variable focus lens |
| US5141678A (en) | 1990-10-10 | 1992-08-25 | Blum Ronald D | Method for forming disposable molds for producing optical quality lenses |
| US5086192A (en) * | 1990-12-14 | 1992-02-04 | Minnesota Mining And Manufacturing Company | Photopolymerizable compositions and photoinitiators therefor |
| JP3193067B2 (en) | 1991-05-23 | 2001-07-30 | 康博 小池 | Method of manufacturing lens for correcting vision |
| US5173381A (en) | 1991-08-05 | 1992-12-22 | Queen's University | Azo polymers for reversible optical storage |
| FR2683918B1 (en) * | 1991-11-19 | 1994-09-09 | Thomson Csf | MATERIAL CONSTITUTING A RIFLE SCOPE AND WEAPON USING THE SAME. |
| US5463084A (en) * | 1992-02-18 | 1995-10-31 | Rensselaer Polytechnic Institute | Photocurable silicone oxetanes |
| EP0593684B1 (en) | 1992-04-21 | 1997-07-16 | Kabi Pharmacia Ophthalmics, Inc. | High refractive index silicone compositions |
| US5444106A (en) | 1992-04-21 | 1995-08-22 | Kabi Pharmacia Ophthalmics, Inc. | High refractive index silicone compositions |
| DE69316792T2 (en) | 1992-06-17 | 1998-05-28 | Nitto Denko Corp | A method of producing polymerization or cross-linked rate-distributed products and a method of producing a lens, lens assembly or optical fiber by this method |
| US5288293A (en) | 1992-09-24 | 1994-02-22 | Donnell Jr Francis E O | In vivo modification of refractive power of an intraocular lens implant |
| US5443506A (en) | 1992-11-18 | 1995-08-22 | Garabet; Antoine L. | Lens with variable optical properties |
| JP3504683B2 (en) * | 1993-04-12 | 2004-03-08 | 日東電工株式会社 | Method of forming lens region, lens and lens array plate |
| RU2033114C1 (en) | 1993-04-22 | 1995-04-20 | Межотраслевой научно-технический комплекс "Микрохирургия глаза" | Artificial crystalline lens |
| US5377176A (en) | 1993-07-14 | 1994-12-27 | Tamarack Storage Devices | Method and apparatus for isolating data storage regions in a thick holographic storage media |
| JP3370762B2 (en) | 1993-11-04 | 2003-01-27 | イー・アイ・デュポン・ドウ・ヌムール・アンド・カンパニー | Film composition and laminated structure containing the composition |
| JPH07281426A (en) | 1994-04-01 | 1995-10-27 | W R Grace & Co | Photosensitive resin composition for forming refractive-index modulated image |
| US5808627A (en) | 1994-04-22 | 1998-09-15 | Apple Computer, Inc. | Method and apparatus for increasing the speed of rendering of objects in a display system |
| EP0689067A3 (en) | 1994-06-22 | 1997-04-09 | Fujitsu Ltd | Method of producing optical waveguide system, optical device and optical coupler employing the same, optical network and optical circuit board |
| DE4431823A1 (en) | 1994-09-07 | 1996-03-14 | Bayer Ag | Process for enhancing information in photoaddressable side chain polymers |
| US5694195A (en) | 1994-09-30 | 1997-12-02 | Signet Armorlite, Inc. | Polyester resin-based high index ophthalmic lenses having improved optical uniformity and/or tintability |
| JPH08101503A (en) | 1994-10-03 | 1996-04-16 | Nippon Paint Co Ltd | Photosensitive composition for three-dimensional hologram recording, recording medium using that and forming method of three-dimensional hologram |
| JP3532621B2 (en) * | 1994-10-03 | 2004-05-31 | 日本ペイント株式会社 | Photosensitive composition for volume hologram recording, recording medium using the same, and method for forming volume hologram |
| US5702846A (en) | 1994-10-03 | 1997-12-30 | Nippon Paint Co. Ltd. | Photosensitive composition for volume hologram recording |
| JPH08101502A (en) | 1994-10-03 | 1996-04-16 | Nippon Paint Co Ltd | Photosensitive composition for three-dimensional hologram recording, recording medium using that and forming method of three-dimensional hologram |
| JPH08101499A (en) | 1994-10-03 | 1996-04-16 | Nippon Paint Co Ltd | Photosensitive composition for volume hologram recording, recording medium using the composition and volume hologram forming method |
| US5744267A (en) | 1994-10-12 | 1998-04-28 | Arizona Board Of Regents Acting For And On Behalf Of University Of Arizona | Azo-dye-doped photorefractive polymer composites for holographic testing and image processing |
| EP0734319B1 (en) | 1994-10-14 | 2002-03-06 | Corneal Laboratoires | Method for making an intraocular implant with a soft lens |
| JP3222026B2 (en) * | 1994-12-26 | 2001-10-22 | 株式会社メニコン | Contact lens material and intraocular lens material |
| FR2731081B1 (en) * | 1995-02-27 | 1997-04-11 | Essilor Int | PROCESS FOR OBTAINING A TRANSPARENT ARTICLE WITH A REFRACTION INDEX |
| TW393498B (en) | 1995-04-04 | 2000-06-11 | Novartis Ag | The preparation and use of Polysiloxane-comprising perfluoroalkyl ethers |
| US5943145A (en) | 1995-05-05 | 1999-08-24 | Lucent Technologies Inc. | Phase distance multiplex holography |
| CA2172643C (en) * | 1995-05-05 | 2000-02-15 | Kevin Curtis | Multiplex holography |
| DE69637663D1 (en) | 1995-07-05 | 2008-10-09 | Yenploy Pty Ltd | OPTICAL STORAGE SYSTEM |
| US5684636A (en) | 1995-08-24 | 1997-11-04 | Lockheed Martin Corporation | Polymer-optical liquid matrix for use as a lens element |
| JPH09106240A (en) * | 1995-10-09 | 1997-04-22 | Toyo Ink Mfg Co Ltd | Hologram recording photosensitive composition, hologram recording medium and production of hologram by using the medium |
| JP3815811B2 (en) * | 1995-10-12 | 2006-08-30 | ダウ・コ−ニング・コ−ポレ−ション | Refractive index modulation element and refractive index modulation method |
| US5984962A (en) | 1996-01-22 | 1999-11-16 | Quantum Vision, Inc. | Adjustable intraocular lens |
| US5728155A (en) | 1996-01-22 | 1998-03-17 | Quantum Solutions, Inc. | Adjustable intraocular lens |
| US5838650A (en) | 1996-06-26 | 1998-11-17 | Lucent Technologies Inc. | Image quality compensation method and apparatus for holographic data storage system |
| US5728156A (en) | 1996-08-06 | 1998-03-17 | Prism Opthalmics, L.L.C. | Prismatic intraocular lenses and related methods of in situ alteration of their optical characteristics |
| DE19631864A1 (en) | 1996-08-07 | 1998-02-12 | Bayer Ag | High sensitivity photoaddressable side group polymers |
| US5777719A (en) | 1996-12-23 | 1998-07-07 | University Of Rochester | Method and apparatus for improving vision and the resolution of retinal images |
| US6174464B1 (en) | 1997-04-21 | 2001-01-16 | Corning Incorporated | Organic photochromic contact lens compositions |
| FR2762840B1 (en) * | 1997-05-02 | 1999-08-13 | Corning Sa | PHOTOCHROMIC COMPOSITIONS, PHOTOCHROMIC COMPOUNDS, (CO) POLYMER MATRICES AND FINISHED PRODUCTS INCORPORATING THEM |
| US5995521A (en) * | 1997-05-16 | 1999-11-30 | New Focus, Inc. | External cavity laser pivot design |
| US20030157414A1 (en) | 1997-11-13 | 2003-08-21 | Pradeep K. Dhal | Holographic medium and process for use thereof |
| JPH11202740A (en) | 1998-01-20 | 1999-07-30 | Fujitsu Ltd | Refractive index distribution forming material and holographic dry plate |
| US5995251A (en) | 1998-07-16 | 1999-11-30 | Siros Technologies, Inc. | Apparatus for holographic data storage |
| US6450642B1 (en) | 1999-01-12 | 2002-09-17 | California Institute Of Technology | Lenses capable of post-fabrication power modification |
| US6271281B1 (en) | 1999-08-26 | 2001-08-07 | Medennium, Inc. | Homopolymers containing stable elasticity inducing crosslinkers and ocular implants made therefrom |
| US6086204A (en) | 1999-09-20 | 2000-07-11 | Magnante; Peter C. | Methods and devices to design and fabricate surfaces on contact lenses and on corneal tissue that correct the eye's optical aberrations |
| US6145432A (en) * | 1999-11-29 | 2000-11-14 | Bellue, Jr.; Wirt E. | Cooking pot |
| JP3677240B2 (en) | 2000-03-20 | 2005-07-27 | カリフォルニア インスティテュート オブ テクノロジー | Application of wavefront sensor to lens with adjustable magnification after manufacturing |
| AU2001259755A1 (en) | 2000-05-10 | 2001-11-20 | California Institute Of Technology | Phase contrast variation of a photo-induced refractive material |
| US6851804B2 (en) * | 2001-12-28 | 2005-02-08 | Jagdish M. Jethmalani | Readjustable optical elements |
-
1999
- 1999-10-08 US US09/416,044 patent/US6450642B1/en not_active Expired - Lifetime
- 1999-10-13 EP EP99951937A patent/EP1139921B1/en not_active Expired - Lifetime
- 1999-10-13 WO PCT/US1999/023728 patent/WO2000041650A1/en active IP Right Grant
- 1999-10-13 MX MXPA01007051A patent/MXPA01007051A/en unknown
- 1999-10-13 DE DE69935449T patent/DE69935449T2/en not_active Expired - Lifetime
- 1999-10-13 CN CNB998163007A patent/CN1306918C/en not_active Expired - Fee Related
- 1999-10-13 JP JP2000593264A patent/JP2004500585A/en not_active Withdrawn
- 1999-10-13 IL IL14424599A patent/IL144245A0/en not_active IP Right Cessation
- 1999-10-13 CA CA002360583A patent/CA2360583A1/en not_active Abandoned
- 1999-10-13 BR BR9916895-2A patent/BR9916895A/en not_active IP Right Cessation
- 1999-10-13 AU AU64267/99A patent/AU766157B2/en not_active Ceased
- 1999-10-13 AT AT99951937T patent/ATE355800T1/en not_active IP Right Cessation
-
2001
- 2001-11-21 US US09/991,560 patent/US20020167735A1/en not_active Abandoned
-
2002
- 2002-06-18 US US10/177,722 patent/US20030090624A1/en not_active Abandoned
- 2002-06-18 US US10/175,552 patent/US7210783B2/en not_active Expired - Lifetime
- 2002-06-18 US US10/176,947 patent/US20030090013A1/en not_active Abandoned
- 2002-07-10 US US10/192,017 patent/US20030048411A1/en not_active Abandoned
- 2002-08-15 US US10/223,086 patent/US6813097B2/en not_active Expired - Lifetime
-
2003
- 2003-02-03 US US10/358,065 patent/US6824266B2/en not_active Expired - Lifetime
-
2006
- 2006-06-16 US US11/454,472 patent/US7837326B2/en not_active Expired - Fee Related
-
2007
- 2007-05-01 US US11/743,119 patent/US7798644B2/en not_active Expired - Fee Related
-
2010
- 2010-09-17 JP JP2010209882A patent/JP5411099B2/en not_active Expired - Lifetime
Cited By (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8752958B2 (en) | 1999-03-01 | 2014-06-17 | Boston Innovative Optics, Inc. | System and method for increasing the depth of focus of the human eye |
| US20060084949A1 (en) * | 2000-03-21 | 2006-04-20 | Peyman Gholam A | Method and apparatus for accommodating intraocular lens |
| US20040049174A1 (en) * | 2000-03-21 | 2004-03-11 | Peyman Gholam A. | Adjustable inlay with multizone polymerization |
| US8162927B2 (en) | 2000-03-21 | 2012-04-24 | Gholam A. Peyman | Method and apparatus for accommodating intraocular lens |
| US20060216329A1 (en) * | 2000-03-21 | 2006-09-28 | Peyman Gholam A | Drug delivery system and method |
| US7001374B2 (en) | 2000-03-21 | 2006-02-21 | Minu, L.L.C. | Adjustable inlay with multizone polymerization |
| US20060261502A1 (en) * | 2000-09-26 | 2006-11-23 | Platt Ben C | Delivery system for post-operative power adjustment of adjustable lens |
| US7105110B2 (en) | 2000-09-26 | 2006-09-12 | Calhoun Vision, Inc. | Delivery system for post-operative power adjustment of adjustable lens |
| US20050192563A1 (en) * | 2000-09-26 | 2005-09-01 | Calhoun Vision, Inc. | Delivery system for post-operative power adjustment of adjustable lens |
| US6905641B2 (en) | 2000-09-26 | 2005-06-14 | Calhoun Vision, Inc. | Delivery system for post-operative power adjustment of adjustable lens |
| US20050182489A1 (en) * | 2001-04-27 | 2005-08-18 | Peyman Gholam A. | Intraocular lens adapted for adjustment via laser after implantation |
| US20050113911A1 (en) * | 2002-10-17 | 2005-05-26 | Peyman Gholam A. | Adjustable intraocular lens for insertion into the capsular bag |
| US9717413B2 (en) * | 2004-04-16 | 2017-08-01 | Senseonics, Incorporated | Biocompatible, human implantable apparatus and method for fully encasing a circuit within a polymer housing |
| US20110255255A1 (en) * | 2004-04-16 | 2011-10-20 | Sensors For Medicine And Science, Inc. | Housing for a circuit that is to be implanted in-vivo and process of making the same |
| US20070031473A1 (en) * | 2005-08-05 | 2007-02-08 | Peyman Gholam A | Drug delivery system and method |
| US20070142909A1 (en) * | 2005-10-27 | 2007-06-21 | Minu Llc | External lens adapted to change refractive properties |
| US7993399B2 (en) | 2005-10-27 | 2011-08-09 | Gholam A. Peyman | External lens adapted to change refractive properties |
| US9681800B2 (en) | 2005-10-27 | 2017-06-20 | The Arizona Board Of Regents On Behalf Of The University Of Arizona | Holographic adaptive see-through phoropter |
| US20070100443A1 (en) * | 2005-10-27 | 2007-05-03 | Peyman Gholam A | Intraocular lens adapted for accommodation via electrical signals |
| US9545303B2 (en) | 2011-12-02 | 2017-01-17 | Acufocus, Inc. | Ocular mask having selective spectral transmission |
| US9204962B2 (en) | 2013-03-13 | 2015-12-08 | Acufocus, Inc. | In situ adjustable optical mask |
| US9603704B2 (en) | 2013-03-13 | 2017-03-28 | Acufocus, Inc. | In situ adjustable optical mask |
| US10350058B2 (en) | 2013-03-13 | 2019-07-16 | Acufocus, Inc. | In situ adjustable optical mask |
| US10939995B2 (en) | 2013-03-13 | 2021-03-09 | Acufocus, Inc. | In situ adjustable optical mask |
| US11771552B2 (en) | 2013-03-13 | 2023-10-03 | Acufocus, Inc. | In situ adjustable optical mask |
| US9427922B2 (en) | 2013-03-14 | 2016-08-30 | Acufocus, Inc. | Process for manufacturing an intraocular lens with an embedded mask |
| US11266495B2 (en) | 2019-10-20 | 2022-03-08 | Rxsight, Inc. | Light adjustable intraocular lens with a modulable absorption front protection layer |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2000041650A1 (en) | 2000-07-20 |
| JP5411099B2 (en) | 2014-02-12 |
| CN1346251A (en) | 2002-04-24 |
| US20030090624A1 (en) | 2003-05-15 |
| US20030093150A1 (en) | 2003-05-15 |
| MXPA01007051A (en) | 2002-04-24 |
| EP1139921A1 (en) | 2001-10-10 |
| US20030151719A1 (en) | 2003-08-14 |
| CA2360583A1 (en) | 2000-07-20 |
| US20070035698A1 (en) | 2007-02-15 |
| JP2011034091A (en) | 2011-02-17 |
| US6824266B2 (en) | 2004-11-30 |
| US7837326B2 (en) | 2010-11-23 |
| BR9916895A (en) | 2002-03-19 |
| US6813097B2 (en) | 2004-11-02 |
| EP1139921B1 (en) | 2007-03-07 |
| US7210783B2 (en) | 2007-05-01 |
| AU6426799A (en) | 2000-08-01 |
| US7798644B2 (en) | 2010-09-21 |
| US20070260311A1 (en) | 2007-11-08 |
| AU766157B2 (en) | 2003-10-09 |
| JP2004500585A (en) | 2004-01-08 |
| US20030090013A1 (en) | 2003-05-15 |
| CN1306918C (en) | 2007-03-28 |
| WO2000041650B1 (en) | 2000-10-19 |
| US20020167735A1 (en) | 2002-11-14 |
| US20030173691A1 (en) | 2003-09-18 |
| DE69935449D1 (en) | 2007-04-19 |
| US6450642B1 (en) | 2002-09-17 |
| IL144245A0 (en) | 2002-05-23 |
| ATE355800T1 (en) | 2007-03-15 |
| DE69935449T2 (en) | 2007-11-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20030048411A1 (en) | Intraoccular lenses capable of in vivo power adjustment and method for same | |
| US9883940B2 (en) | Multilens intraocular system implantation with injectable accommodation material | |
| US9005282B2 (en) | Intraocular lens system with injectable accommodation material | |
| US8361353B2 (en) | Method for making novel compositions capable of post fabrication modification | |
| AU2006202346B2 (en) | Methods of pre-selecting a polymerizable fluid formed into an introcular lens | |
| US7156101B2 (en) | Methods of implanting an intraocular lens | |
| US20050246018A1 (en) | Injectable accommodation composition | |
| US20070129802A1 (en) | Composition and method for producing shapable implants in vivo and implants produced thereby | |
| US20050099597A1 (en) | Light adjustable multifocal lenses | |
| AU2002336002A1 (en) | Composition and method for producing shapable implants in vivo and implants produced thereby | |
| AU2001262319A1 (en) | Methods of pre-selecting a polymerizable fluid formed into an intraocular lens | |
| SG174010A1 (en) | Eye treatment | |
| WO2003011194A1 (en) | Intraoccular lenses with power adjustability in vivo | |
| WO2007106796A2 (en) | Multilens intraocular lens system with injectable accommodation material |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: CALIFORNIA INSTITUTE OF TECHNOLOGY, CALIFORNIA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:JETHMALANI, JAGDISH M.;MALONEY, ROBERT K.;GRUBBS, ROBERT H.;AND OTHERS;REEL/FRAME:013511/0675;SIGNING DATES FROM 20020821 TO 20020826 Owner name: REGENT OF THE UNIVERSITY CALIFORNIA OF THE, CALIFO Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:SCHWARTZ, DANIEL M.;REEL/FRAME:013511/0590 Effective date: 20020808 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |