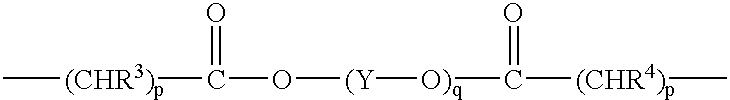US20030130390A1 - Transparent thermoplastischic composition - Google Patents
Transparent thermoplastischic composition Download PDFInfo
- Publication number
- US20030130390A1 US20030130390A1 US10/296,340 US29634002A US2003130390A1 US 20030130390 A1 US20030130390 A1 US 20030130390A1 US 29634002 A US29634002 A US 29634002A US 2003130390 A1 US2003130390 A1 US 2003130390A1
- Authority
- US
- United States
- Prior art keywords
- sheets
- composition according
- product
- polycarbonate
- transparent thermoplastic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 57
- 150000001875 compounds Chemical class 0.000 claims abstract description 21
- 229920000642 polymer Polymers 0.000 claims abstract description 19
- 229920006352 transparent thermoplastic Polymers 0.000 claims abstract description 12
- 229910052739 hydrogen Inorganic materials 0.000 claims abstract description 6
- 229910052736 halogen Inorganic materials 0.000 claims abstract description 5
- 150000002367 halogens Chemical class 0.000 claims abstract description 5
- 125000000217 alkyl group Chemical group 0.000 claims abstract description 4
- 125000004093 cyano group Chemical group *C#N 0.000 claims abstract description 4
- 229920000515 polycarbonate Polymers 0.000 claims description 33
- 239000004417 polycarbonate Substances 0.000 claims description 33
- 239000006096 absorbing agent Substances 0.000 claims description 23
- -1 polyethylene terephthalate Polymers 0.000 claims description 23
- 238000001125 extrusion Methods 0.000 claims description 12
- 229920000728 polyester Polymers 0.000 claims description 7
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 6
- 229930195729 fatty acid Natural products 0.000 claims description 6
- 239000000194 fatty acid Substances 0.000 claims description 6
- 229920003023 plastic Polymers 0.000 claims description 6
- 239000004033 plastic Substances 0.000 claims description 6
- 229920001634 Copolyester Polymers 0.000 claims description 5
- 229920000139 polyethylene terephthalate Polymers 0.000 claims description 5
- 239000005020 polyethylene terephthalate Substances 0.000 claims description 5
- 239000007787 solid Substances 0.000 claims description 5
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 claims description 4
- ORLQHILJRHBSAY-UHFFFAOYSA-N [1-(hydroxymethyl)cyclohexyl]methanol Chemical compound OCC1(CO)CCCCC1 ORLQHILJRHBSAY-UHFFFAOYSA-N 0.000 claims description 4
- 150000001298 alcohols Chemical class 0.000 claims description 4
- 229920001707 polybutylene terephthalate Polymers 0.000 claims description 4
- 239000004695 Polyether sulfone Substances 0.000 claims description 2
- 239000004698 Polyethylene Substances 0.000 claims description 2
- 239000004743 Polypropylene Substances 0.000 claims description 2
- 239000004793 Polystyrene Substances 0.000 claims description 2
- OCKWAZCWKSMKNC-UHFFFAOYSA-N [3-octadecanoyloxy-2,2-bis(octadecanoyloxymethyl)propyl] octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(COC(=O)CCCCCCCCCCCCCCCCC)(COC(=O)CCCCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCCCC OCKWAZCWKSMKNC-UHFFFAOYSA-N 0.000 claims description 2
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 claims description 2
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 claims description 2
- 230000009931 harmful effect Effects 0.000 claims description 2
- 229920001483 poly(ethyl methacrylate) polymer Polymers 0.000 claims description 2
- 229920003229 poly(methyl methacrylate) Polymers 0.000 claims description 2
- 229920002285 poly(styrene-co-acrylonitrile) Polymers 0.000 claims description 2
- 229920002492 poly(sulfone) Polymers 0.000 claims description 2
- 229920006393 polyether sulfone Polymers 0.000 claims description 2
- 229920000573 polyethylene Polymers 0.000 claims description 2
- 239000004926 polymethyl methacrylate Substances 0.000 claims description 2
- 229920001155 polypropylene Polymers 0.000 claims description 2
- 229920002223 polystyrene Polymers 0.000 claims description 2
- 238000010422 painting Methods 0.000 claims 1
- 230000005540 biological transmission Effects 0.000 abstract description 7
- 238000002360 preparation method Methods 0.000 abstract 1
- 238000009757 thermoplastic moulding Methods 0.000 abstract 1
- 239000000047 product Substances 0.000 description 16
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 14
- IISBACLAFKSPIT-UHFFFAOYSA-N bisphenol A Chemical compound C=1C=C(O)C=CC=1C(C)(C)C1=CC=C(O)C=C1 IISBACLAFKSPIT-UHFFFAOYSA-N 0.000 description 11
- FMRHJJZUHUTGKE-UHFFFAOYSA-N Ethylhexyl salicylate Chemical compound CCCCC(CC)COC(=O)C1=CC=CC=C1O FMRHJJZUHUTGKE-UHFFFAOYSA-N 0.000 description 10
- 229930185605 Bisphenol Natural products 0.000 description 9
- 238000000034 method Methods 0.000 description 9
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 description 8
- 238000004519 manufacturing process Methods 0.000 description 8
- VPWNQTHUCYMVMZ-UHFFFAOYSA-N 4,4'-sulfonyldiphenol Chemical class C1=CC(O)=CC=C1S(=O)(=O)C1=CC=C(O)C=C1 VPWNQTHUCYMVMZ-UHFFFAOYSA-N 0.000 description 7
- 239000003795 chemical substances by application Substances 0.000 description 6
- 239000000243 solution Substances 0.000 description 6
- 0 [1*]C1=C([2*])C([3*])=C([4*])C([5*])=C1/C(C1=C([10*])C([9*])=C([8*])C([7*])=C1[6*])=C(\[N+]#[C-])C(=O)OCC(COC(=O)/C(C#N)=C(/C1=C([25*])C([24*])=C([23*])C([22*])=C1[21*])C1=C([26*])C([27*])=C([28*])C([29*])=C1[30*])(COC(=O)/C(C#N)=C(/C1=C([35*])C([34*])=C([33*])C([32*])=C1[31*])C1=C([40*])C([39*])=C([38*])C([37*])=C1[36*])COC(=O)/C([N+]#[C-])=C(/C1=C([15*])C([14*])=C([13*])C([12*])=C1[11*])C1=C([20*])C([19*])=C([18*])C([17*])=C1[16*] Chemical compound [1*]C1=C([2*])C([3*])=C([4*])C([5*])=C1/C(C1=C([10*])C([9*])=C([8*])C([7*])=C1[6*])=C(\[N+]#[C-])C(=O)OCC(COC(=O)/C(C#N)=C(/C1=C([25*])C([24*])=C([23*])C([22*])=C1[21*])C1=C([26*])C([27*])=C([28*])C([29*])=C1[30*])(COC(=O)/C(C#N)=C(/C1=C([35*])C([34*])=C([33*])C([32*])=C1[31*])C1=C([40*])C([39*])=C([38*])C([37*])=C1[36*])COC(=O)/C([N+]#[C-])=C(/C1=C([15*])C([14*])=C([13*])C([12*])=C1[11*])C1=C([20*])C([19*])=C([18*])C([17*])=C1[16*] 0.000 description 5
- 239000000654 additive Substances 0.000 description 5
- 239000006085 branching agent Substances 0.000 description 5
- 239000000155 melt Substances 0.000 description 5
- 239000003381 stabilizer Substances 0.000 description 5
- 238000012696 Interfacial polycondensation Methods 0.000 description 4
- KKEYFWRCBNTPAC-UHFFFAOYSA-N Terephthalic acid Chemical compound OC(=O)C1=CC=C(C(O)=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-N 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 238000001746 injection moulding Methods 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 238000005809 transesterification reaction Methods 0.000 description 4
- 238000004383 yellowing Methods 0.000 description 4
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 230000006750 UV protection Effects 0.000 description 3
- 239000002216 antistatic agent Substances 0.000 description 3
- 125000003118 aryl group Chemical group 0.000 description 3
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- AQSJGOWTSHOLKH-UHFFFAOYSA-N phosphite(3-) Chemical class [O-]P([O-])[O-] AQSJGOWTSHOLKH-UHFFFAOYSA-N 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 2
- RFFLAFLAYFXFSW-UHFFFAOYSA-N 1,2-dichlorobenzene Chemical compound ClC1=CC=CC=C1Cl RFFLAFLAYFXFSW-UHFFFAOYSA-N 0.000 description 2
- UIAFKZKHHVMJGS-UHFFFAOYSA-N 2,4-dihydroxybenzoic acid Chemical compound OC(=O)C1=CC=C(O)C=C1O UIAFKZKHHVMJGS-UHFFFAOYSA-N 0.000 description 2
- CJWNFAKWHDOUKL-UHFFFAOYSA-N 2-(2-phenylpropan-2-yl)phenol Chemical compound C=1C=CC=C(O)C=1C(C)(C)C1=CC=CC=C1 CJWNFAKWHDOUKL-UHFFFAOYSA-N 0.000 description 2
- XKZQKPRCPNGNFR-UHFFFAOYSA-N 2-(3-hydroxyphenyl)phenol Chemical compound OC1=CC=CC(C=2C(=CC=CC=2)O)=C1 XKZQKPRCPNGNFR-UHFFFAOYSA-N 0.000 description 2
- ZEKCYPANSOJWDH-UHFFFAOYSA-N 3,3-bis(4-hydroxy-3-methylphenyl)-1H-indol-2-one Chemical compound C1=C(O)C(C)=CC(C2(C3=CC=CC=C3NC2=O)C=2C=C(C)C(O)=CC=2)=C1 ZEKCYPANSOJWDH-UHFFFAOYSA-N 0.000 description 2
- BRPSWMCDEYMRPE-UHFFFAOYSA-N 4-[1,1-bis(4-hydroxyphenyl)ethyl]phenol Chemical compound C=1C=C(O)C=CC=1C(C=1C=CC(O)=CC=1)(C)C1=CC=C(O)C=C1 BRPSWMCDEYMRPE-UHFFFAOYSA-N 0.000 description 2
- UMPGNGRIGSEMTC-UHFFFAOYSA-N 4-[1-(4-hydroxyphenyl)-3,3,5-trimethylcyclohexyl]phenol Chemical compound C1C(C)CC(C)(C)CC1(C=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 UMPGNGRIGSEMTC-UHFFFAOYSA-N 0.000 description 2
- QHPQWRBYOIRBIT-UHFFFAOYSA-N 4-tert-butylphenol Chemical compound CC(C)(C)C1=CC=C(O)C=C1 QHPQWRBYOIRBIT-UHFFFAOYSA-N 0.000 description 2
- SDDLEVPIDBLVHC-UHFFFAOYSA-N Bisphenol Z Chemical compound C1=CC(O)=CC=C1C1(C=2C=CC(O)=CC=2)CCCCC1 SDDLEVPIDBLVHC-UHFFFAOYSA-N 0.000 description 2
- 125000005915 C6-C14 aryl group Chemical group 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- QMKYBPDZANOJGF-UHFFFAOYSA-N benzene-1,3,5-tricarboxylic acid Chemical compound OC(=O)C1=CC(C(O)=O)=CC(C(O)=O)=C1 QMKYBPDZANOJGF-UHFFFAOYSA-N 0.000 description 2
- 150000001565 benzotriazoles Chemical class 0.000 description 2
- 239000003054 catalyst Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- 125000000753 cycloalkyl group Chemical group 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 150000004665 fatty acids Chemical class 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 238000005286 illumination Methods 0.000 description 2
- 238000010348 incorporation Methods 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- QQVIHTHCMHWDBS-UHFFFAOYSA-N isophthalic acid Chemical compound OC(=O)C1=CC=CC(C(O)=O)=C1 QQVIHTHCMHWDBS-UHFFFAOYSA-N 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 239000011368 organic material Substances 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- XNGIFLGASWRNHJ-UHFFFAOYSA-N phthalic acid Chemical compound OC(=O)C1=CC=CC=C1C(O)=O XNGIFLGASWRNHJ-UHFFFAOYSA-N 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229920002959 polymer blend Polymers 0.000 description 2
- 230000003019 stabilising effect Effects 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- UXWKVPJOPVIIRU-UHFFFAOYSA-N (6-methylsulfanylpyridin-3-yl)boronic acid Chemical compound CSC1=CC=C(B(O)O)C=N1 UXWKVPJOPVIIRU-UHFFFAOYSA-N 0.000 description 1
- SCYULBFZEHDVBN-UHFFFAOYSA-N 1,1-Dichloroethane Chemical class CC(Cl)Cl SCYULBFZEHDVBN-UHFFFAOYSA-N 0.000 description 1
- YIYBRXKMQFDHSM-UHFFFAOYSA-N 2,2'-Dihydroxybenzophenone Chemical class OC1=CC=CC=C1C(=O)C1=CC=CC=C1O YIYBRXKMQFDHSM-UHFFFAOYSA-N 0.000 description 1
- DFOLZQISJZKWBT-UHFFFAOYSA-N 2,3-dihydro-1h-indene;phenol Chemical class OC1=CC=CC=C1.OC1=CC=CC=C1.C1=CC=C2CCCC2=C1 DFOLZQISJZKWBT-UHFFFAOYSA-N 0.000 description 1
- VPVTXVHUJHGOCM-UHFFFAOYSA-N 2,4-bis[2-(4-hydroxyphenyl)propan-2-yl]phenol Chemical compound C=1C=C(O)C(C(C)(C)C=2C=CC(O)=CC=2)=CC=1C(C)(C)C1=CC=C(O)C=C1 VPVTXVHUJHGOCM-UHFFFAOYSA-N 0.000 description 1
- MAQOZOILPAMFSW-UHFFFAOYSA-N 2,6-bis[(2-hydroxy-5-methylphenyl)methyl]-4-methylphenol Chemical compound CC1=CC=C(O)C(CC=2C(=C(CC=3C(=CC=C(C)C=3)O)C=C(C)C=2)O)=C1 MAQOZOILPAMFSW-UHFFFAOYSA-N 0.000 description 1
- JLZIIHMTTRXXIN-UHFFFAOYSA-N 2-(2-hydroxy-4-methoxybenzoyl)benzoic acid Chemical compound OC1=CC(OC)=CC=C1C(=O)C1=CC=CC=C1C(O)=O JLZIIHMTTRXXIN-UHFFFAOYSA-N 0.000 description 1
- VXHYVVAUHMGCEX-UHFFFAOYSA-N 2-(2-hydroxyphenoxy)phenol Chemical class OC1=CC=CC=C1OC1=CC=CC=C1O VXHYVVAUHMGCEX-UHFFFAOYSA-N 0.000 description 1
- QUWAJPZDCZDTJS-UHFFFAOYSA-N 2-(2-hydroxyphenyl)sulfonylphenol Chemical class OC1=CC=CC=C1S(=O)(=O)C1=CC=CC=C1O QUWAJPZDCZDTJS-UHFFFAOYSA-N 0.000 description 1
- IYAZLDLPUNDVAG-UHFFFAOYSA-N 2-(benzotriazol-2-yl)-4-(2,4,4-trimethylpentan-2-yl)phenol Chemical compound CC(C)(C)CC(C)(C)C1=CC=C(O)C(N2N=C3C=CC=CC3=N2)=C1 IYAZLDLPUNDVAG-UHFFFAOYSA-N 0.000 description 1
- ZSSVCEUEVMALRD-UHFFFAOYSA-N 2-[4,6-bis(2,4-dimethylphenyl)-1,3,5-triazin-2-yl]-5-(octyloxy)phenol Chemical compound OC1=CC(OCCCCCCCC)=CC=C1C1=NC(C=2C(=CC(C)=CC=2)C)=NC(C=2C(=CC(C)=CC=2)C)=N1 ZSSVCEUEVMALRD-UHFFFAOYSA-N 0.000 description 1
- VADKRMSMGWJZCF-UHFFFAOYSA-N 2-bromophenol Chemical compound OC1=CC=CC=C1Br VADKRMSMGWJZCF-UHFFFAOYSA-N 0.000 description 1
- ISPYQTSUDJAMAB-UHFFFAOYSA-N 2-chlorophenol Chemical compound OC1=CC=CC=C1Cl ISPYQTSUDJAMAB-UHFFFAOYSA-N 0.000 description 1
- QTWJRLJHJPIABL-UHFFFAOYSA-N 2-methylphenol;3-methylphenol;4-methylphenol Chemical compound CC1=CC=C(O)C=C1.CC1=CC=CC(O)=C1.CC1=CC=CC=C1O QTWJRLJHJPIABL-UHFFFAOYSA-N 0.000 description 1
- NEQFBGHQPUXOFH-UHFFFAOYSA-N 4-(4-carboxyphenyl)benzoic acid Chemical compound C1=CC(C(=O)O)=CC=C1C1=CC=C(C(O)=O)C=C1 NEQFBGHQPUXOFH-UHFFFAOYSA-N 0.000 description 1
- XJGTVJRTDRARGO-UHFFFAOYSA-N 4-[2-(4-hydroxyphenyl)propan-2-yl]benzene-1,3-diol Chemical compound C=1C=C(O)C=C(O)C=1C(C)(C)C1=CC=C(O)C=C1 XJGTVJRTDRARGO-UHFFFAOYSA-N 0.000 description 1
- PVFQHGDIOXNKIC-UHFFFAOYSA-N 4-[2-[3-[2-(4-hydroxyphenyl)propan-2-yl]phenyl]propan-2-yl]phenol Chemical compound C=1C=CC(C(C)(C)C=2C=CC(O)=CC=2)=CC=1C(C)(C)C1=CC=C(O)C=C1 PVFQHGDIOXNKIC-UHFFFAOYSA-N 0.000 description 1
- JHSDIILQGDBNPD-UHFFFAOYSA-N 4-[2-[4-[tris[4-[2-(4-hydroxyphenyl)propan-2-yl]phenoxy]methoxy]phenyl]propan-2-yl]phenol Chemical compound C=1C=C(OC(OC=2C=CC(=CC=2)C(C)(C)C=2C=CC(O)=CC=2)(OC=2C=CC(=CC=2)C(C)(C)C=2C=CC(O)=CC=2)OC=2C=CC(=CC=2)C(C)(C)C=2C=CC(O)=CC=2)C=CC=1C(C)(C)C1=CC=C(O)C=C1 JHSDIILQGDBNPD-UHFFFAOYSA-N 0.000 description 1
- RQTDWDATSAVLOR-UHFFFAOYSA-N 4-[3,5-bis(4-hydroxyphenyl)phenyl]phenol Chemical compound C1=CC(O)=CC=C1C1=CC(C=2C=CC(O)=CC=2)=CC(C=2C=CC(O)=CC=2)=C1 RQTDWDATSAVLOR-UHFFFAOYSA-N 0.000 description 1
- MIJYTDQAOVQRRT-UHFFFAOYSA-N 4-[4,6-bis(4-hydroxyphenyl)-4,6-dimethylhept-2-en-2-yl]phenol Chemical compound C=1C=C(O)C=CC=1C(C)=CC(C)(C=1C=CC(O)=CC=1)CC(C)(C)C1=CC=C(O)C=C1 MIJYTDQAOVQRRT-UHFFFAOYSA-N 0.000 description 1
- CIEGINNQDIULCT-UHFFFAOYSA-N 4-[4,6-bis(4-hydroxyphenyl)-4,6-dimethylheptan-2-yl]phenol Chemical compound C=1C=C(O)C=CC=1C(C)CC(C)(C=1C=CC(O)=CC=1)CC(C)(C)C1=CC=C(O)C=C1 CIEGINNQDIULCT-UHFFFAOYSA-N 0.000 description 1
- IQNDEQHJTOJHAK-UHFFFAOYSA-N 4-[4-[2-[4,4-bis(4-hydroxyphenyl)cyclohexyl]propan-2-yl]-1-(4-hydroxyphenyl)cyclohexyl]phenol Chemical compound C1CC(C=2C=CC(O)=CC=2)(C=2C=CC(O)=CC=2)CCC1C(C)(C)C(CC1)CCC1(C=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 IQNDEQHJTOJHAK-UHFFFAOYSA-N 0.000 description 1
- LIDWAYDGZUAJEG-UHFFFAOYSA-N 4-[bis(4-hydroxyphenyl)-phenylmethyl]phenol Chemical compound C1=CC(O)=CC=C1C(C=1C=CC(O)=CC=1)(C=1C=CC(O)=CC=1)C1=CC=CC=C1 LIDWAYDGZUAJEG-UHFFFAOYSA-N 0.000 description 1
- BOCLKUCIZOXUEY-UHFFFAOYSA-N 4-[tris(4-hydroxyphenyl)methyl]phenol Chemical compound C1=CC(O)=CC=C1C(C=1C=CC(O)=CC=1)(C=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 BOCLKUCIZOXUEY-UHFFFAOYSA-N 0.000 description 1
- VOWWYDCFAISREI-UHFFFAOYSA-N Bisphenol AP Chemical compound C=1C=C(O)C=CC=1C(C=1C=CC(O)=CC=1)(C)C1=CC=CC=C1 VOWWYDCFAISREI-UHFFFAOYSA-N 0.000 description 1
- VDHWJMYHIPJKBQ-UHFFFAOYSA-N CC.CC.CC.CC.OC1=CC=C(CC2=CC=C(O)C(N3/N=C4/C=CC=C/C4=N/3)=C2)C=C1N1N=C2C=CC=CC2=N1 Chemical compound CC.CC.CC.CC.OC1=CC=C(CC2=CC=C(O)C(N3/N=C4/C=CC=C/C4=N/3)=C2)C=C1N1N=C2C=CC=CC2=N1 VDHWJMYHIPJKBQ-UHFFFAOYSA-N 0.000 description 1
- MQHVPSNEKYHWPC-UHFFFAOYSA-N CCC(=O)CCOC(=O)CC Chemical compound CCC(=O)CCOC(=O)CC MQHVPSNEKYHWPC-UHFFFAOYSA-N 0.000 description 1
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 1
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical class S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 229920004061 Makrolon® 3108 Polymers 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 description 1
- HTLZVHNRZJPSMI-UHFFFAOYSA-N N-ethylpiperidine Chemical compound CCN1CCCCC1 HTLZVHNRZJPSMI-UHFFFAOYSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- JKIJEFPNVSHHEI-UHFFFAOYSA-N Phenol, 2,4-bis(1,1-dimethylethyl)-, phosphite (3:1) Chemical compound CC(C)(C)C1=CC(C(C)(C)C)=CC=C1OP(OC=1C(=CC(=CC=1)C(C)(C)C)C(C)(C)C)OC1=CC=C(C(C)(C)C)C=C1C(C)(C)C JKIJEFPNVSHHEI-UHFFFAOYSA-N 0.000 description 1
- JPYHHZQJCSQRJY-UHFFFAOYSA-N Phloroglucinol Natural products CCC=CCC=CCC=CCC=CCCCCC(=O)C1=C(O)C=C(O)C=C1O JPYHHZQJCSQRJY-UHFFFAOYSA-N 0.000 description 1
- YGYAWVDWMABLBF-UHFFFAOYSA-N Phosgene Chemical compound ClC(Cl)=O YGYAWVDWMABLBF-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- BEIOEBMXPVYLRY-UHFFFAOYSA-N [4-[4-bis(2,4-ditert-butylphenoxy)phosphanylphenyl]phenyl]-bis(2,4-ditert-butylphenoxy)phosphane Chemical compound CC(C)(C)C1=CC(C(C)(C)C)=CC=C1OP(C=1C=CC(=CC=1)C=1C=CC(=CC=1)P(OC=1C(=CC(=CC=1)C(C)(C)C)C(C)(C)C)OC=1C(=CC(=CC=1)C(C)(C)C)C(C)(C)C)OC1=CC=C(C(C)(C)C)C=C1C(C)(C)C BEIOEBMXPVYLRY-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 150000001350 alkyl halides Chemical class 0.000 description 1
- 150000008051 alkyl sulfates Chemical class 0.000 description 1
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 150000001449 anionic compounds Chemical class 0.000 description 1
- 238000000149 argon plasma sintering Methods 0.000 description 1
- 125000003710 aryl alkyl group Chemical group 0.000 description 1
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 description 1
- 239000012964 benzotriazole Substances 0.000 description 1
- KCXMKQUNVWSEMD-UHFFFAOYSA-N benzyl chloride Chemical compound ClCC1=CC=CC=C1 KCXMKQUNVWSEMD-UHFFFAOYSA-N 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 229940114055 beta-resorcylic acid Drugs 0.000 description 1
- FQUNFJULCYSSOP-UHFFFAOYSA-N bisoctrizole Chemical compound N1=C2C=CC=CC2=NN1C1=CC(C(C)(C)CC(C)(C)C)=CC(CC=2C(=C(C=C(C=2)C(C)(C)CC(C)(C)C)N2N=C3C=CC=CC3=N2)O)=C1O FQUNFJULCYSSOP-UHFFFAOYSA-N 0.000 description 1
- 229920000402 bisphenol A polycarbonate polymer Polymers 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 229950005499 carbon tetrachloride Drugs 0.000 description 1
- 125000005587 carbonate group Chemical group 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical compound OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 description 1
- 150000007942 carboxylates Chemical class 0.000 description 1
- 150000001767 cationic compounds Chemical class 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 229960001701 chloroform Drugs 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 229930003836 cresol Natural products 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- MGNCLNQXLYJVJD-UHFFFAOYSA-N cyanuric chloride Chemical compound ClC1=NC(Cl)=NC(Cl)=N1 MGNCLNQXLYJVJD-UHFFFAOYSA-N 0.000 description 1
- 150000001925 cycloalkenes Chemical class 0.000 description 1
- 238000000151 deposition Methods 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- IEJIGPNLZYLLBP-UHFFFAOYSA-N dimethyl carbonate Chemical compound COC(=O)OC IEJIGPNLZYLLBP-UHFFFAOYSA-N 0.000 description 1
- ROORDVPLFPIABK-UHFFFAOYSA-N diphenyl carbonate Chemical compound C=1C=CC=CC=1OC(=O)OC1=CC=CC=C1 ROORDVPLFPIABK-UHFFFAOYSA-N 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 150000002118 epoxides Chemical class 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 125000004464 hydroxyphenyl group Chemical group 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 239000001023 inorganic pigment Substances 0.000 description 1
- 238000010030 laminating Methods 0.000 description 1
- 239000004611 light stabiliser Substances 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- SNMVRZFUUCLYTO-UHFFFAOYSA-N n-propyl chloride Chemical class CCCCl SNMVRZFUUCLYTO-UHFFFAOYSA-N 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- WXZMFSXDPGVJKK-UHFFFAOYSA-N pentaerythritol Chemical compound OCC(CO)(CO)CO WXZMFSXDPGVJKK-UHFFFAOYSA-N 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- QCDYQQDYXPDABM-UHFFFAOYSA-N phloroglucinol Chemical compound OC1=CC(O)=CC(O)=C1 QCDYQQDYXPDABM-UHFFFAOYSA-N 0.000 description 1
- 229960001553 phloroglucinol Drugs 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 150000003003 phosphines Chemical class 0.000 description 1
- XYFCBTPGUUZFHI-UHFFFAOYSA-O phosphonium Chemical compound [PH4+] XYFCBTPGUUZFHI-UHFFFAOYSA-O 0.000 description 1
- OJMIONKXNSYLSR-UHFFFAOYSA-N phosphorous acid Chemical compound OP(O)O OJMIONKXNSYLSR-UHFFFAOYSA-N 0.000 description 1
- 239000002985 plastic film Substances 0.000 description 1
- 229920005644 polyethylene terephthalate glycol copolymer Polymers 0.000 description 1
- 229920000193 polymethacrylate Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 238000009747 press moulding Methods 0.000 description 1
- 230000004224 protection Effects 0.000 description 1
- 125000001453 quaternary ammonium group Chemical group 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 230000003678 scratch resistant effect Effects 0.000 description 1
- 238000009987 spinning Methods 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 238000003892 spreading Methods 0.000 description 1
- 238000004544 sputter deposition Methods 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 238000004381 surface treatment Methods 0.000 description 1
- KKEYFWRCBNTPAC-UHFFFAOYSA-L terephthalate(2-) Chemical compound [O-]C(=O)C1=CC=C(C([O-])=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-L 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical compound ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 1
- 238000003856 thermoforming Methods 0.000 description 1
- 229920001169 thermoplastic Polymers 0.000 description 1
- 239000004416 thermosoftening plastic Substances 0.000 description 1
- 238000011282 treatment Methods 0.000 description 1
- 150000003918 triazines Chemical class 0.000 description 1
- IMFACGCPASFAPR-UHFFFAOYSA-N tributylamine Chemical compound CCCCN(CCCC)CCCC IMFACGCPASFAPR-UHFFFAOYSA-N 0.000 description 1
- HVLLSGMXQDNUAL-UHFFFAOYSA-N triphenyl phosphite Chemical class C=1C=CC=CC=1OP(OC=1C=CC=CC=1)OC1=CC=CC=C1 HVLLSGMXQDNUAL-UHFFFAOYSA-N 0.000 description 1
- WGKLOLBTFWFKOD-UHFFFAOYSA-N tris(2-nonylphenyl) phosphite Chemical compound CCCCCCCCCC1=CC=CC=C1OP(OC=1C(=CC=CC=1)CCCCCCCCC)OC1=CC=CC=C1CCCCCCCCC WGKLOLBTFWFKOD-UHFFFAOYSA-N 0.000 description 1
- 229910052724 xenon Inorganic materials 0.000 description 1
- FHNFHKCVQCLJFQ-UHFFFAOYSA-N xenon atom Chemical compound [Xe] FHNFHKCVQCLJFQ-UHFFFAOYSA-N 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- 150000003738 xylenes Chemical class 0.000 description 1
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/18—Layered products comprising a layer of synthetic resin characterised by the use of special additives
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/315—Compounds containing carbon-to-nitrogen triple bonds
Definitions
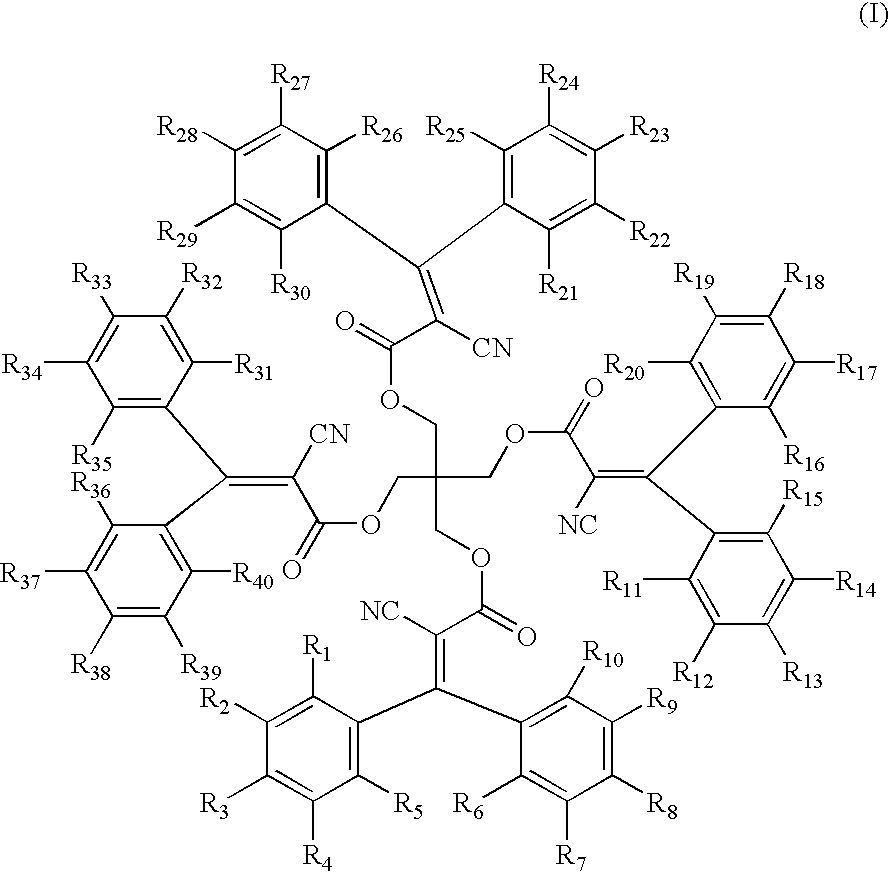
- the present invention relates to a composition containing a transparent thermoplastic polymer and compounds according to formula (I)
- R 1 to R 40 are the same or different and are selected from the group consisting of H, alkyl, halogen and —CN,
- Polycarbonate sheets are known, e.g. from EP-A 0 110 221, and are provided for a large number of applications. Production takes place e.g. by extrusion of compositions containing polycarbonate and optionally co-extrusion with compositions containing polycarbonate that can contain an elevated proportion of UV absorbers.
- compositions containing polycarbonate such as e.g. spectacle lenses and plastic headlight lenses
- Automobile glazing can be made by either injection moulding or extrusion, as desired.
- the polycarbonate normally incorporates at least one UV stabiliser.
- the polycarbonate In the case of transparent glazing, increased haze of the sheet leads to poorer visibility of objects behind it. The haze effect is optically more noticeable the thicker the sheet. Low haze is particularly important, for example, in automobile glazing.
- DE-A 1 670 951 teaches that UV absorbers based, e.g., on substituted benzotriazoles are used for stabilising polycarbonate against yellowing under the action of UV light.
- EP-A 0 320 632 teaches that the polycarbonate sheets should incorporate a co-extrusion layer containing low-volatility UV absorbers, especially dimeric benzotriazoles, such as e.g. Tinuvin® 360 (bis[2-hydroxy-5-tert-octyl-3-(benzotriazol-2-yl)phenyl]methane), a product of Ciba Spezialitätenchemie, Basel, Switzerland, in a sufficiently high concentration.
- Tinuvin® 360 bis[2-hydroxy-5-tert-octyl-3-(benzotriazol-2-yl)phenyl]methane
- WO 96/15102 describes special UV absorbers, including those according to formula (I), which act as a light stabiliser or a stabiliser for organic materials, such as e.g. plastics.
- WO 96/15102 teaches that a content of 0.01 to 10 wt. % of the UV absorbers described there bring about UV protection in organic materials. However, nothing is said about the quality of the UV protection in polycarbonate. Also, no teaching is disclosed as to how low-haze compositions can be obtained.
- the present invention is based on the object of providing compositions that are stable towards UV radiation and exhibit high light transmission and low haze. Furthermore, products made from these compositions are to be provided.
- R 1 to R 40 are the same or different and are selected from the group consisting of H, alkyl, halogen and —CN,
- composition according to the invention preferably contains 0.08 to 0.6 wt. %, particularly preferably 0.1 to 0.3 wt. %, of the compounds according to formula (I).
- R 1 to R 40 preferably equal H (hydrogen).
- the transparent thermoplastic polymer according to the invention is preferably selected from the group consisting of polycarbonate, polymethyl methacrylate, polyethyl methacrylate, polystyrene, polysulfone, styrene-acrylonitrile copolymer, polyester, polyethylene terephthalate, polybutylene terephthalate, copolyesters of polyethylene terephthalate with cyclohexanedimethanol, copolyesters of polybutylene terephthalate with cyclohexanedimethanol, polyether sulfone, polyethylene, polypropylene and mixtures of the above polymers, those polymers and mixtures that can be processed into highly transparent, crystal clear products being preferred.
- the transparent thermoplastic polymer polycarbonate is especially preferred.
- Polycarbonates preferred according to the invention are polycarbonates based on bisphenol A, especially bisphenol A homopolycarbonate and copolycarbonates based on bisphenol A and 1,1-bis(4-hydroxyphenyl)-3,3,5-trimethylcyclohexane.
- Particularly suitable transparent thermoplastic polymers are also copolycarbonates based on bisphenols, poly- or copolyacrylates and poly- or copolymethacrylates, such as e.g. poly- or copolymethyl methacrylate, copolymers with styrene, such as e.g. transparent polystyrene-acrylonitrile (SAN), and also transparent cycloolefins, poly- or copolycondensates of terephthalic acid, such as e.g. poly- or copolyethylene terephthalate (PET or CoPET or PETG).
- SAN transparent polystyrene-acrylonitrile
- polyesters and copolyesters are described in EP-A 0 678 376, EP-A 0 595 413 and U.S. Pat. No. 6,096,854.
- compositions according to the invention preferably also contain 0.01 to 1 wt. %, particularly preferably 0.04 to 0.7 wt. %, pentaerythritol tetrastearate or glycerol monostearate or fatty acid esters of Guerbet alcohols or mixtures thereof.
- Products selected from the group consisting of sheets, solid sheets, multi wall sheets, corrugated sheets, glazing panels, greenhouses, conservatories, bus shelters, advertising panels, signs, safety screens, automobile glazing, windows, roofing, plastic headlight lenses and spectacle lenses are preferred.
- Products that are multi-layer, and in which at least one layer contains a sufficiently high content of a UV absorber that the layers below it are protected from the harmful effects of UV light are also preferred.
- a preferred embodiment of the present invention is provided by the fact that the UV absorber contained in at least one layer is a compound according to formula (I).
- Another preferred embodiment of the present invention is provided by the fact that the UV absorber contained in at least one layer is another compound.
- the transparent thermoplastic polymer is a polymer blend consisting of at least 20 wt. % polycarbonate, the polymer blend also containing polyesters or polymethacrylates or both as blend partners in addition to polycarbonate.
- the polycarbonates according to the invention are homopolycarbonates, copolycarbonates and thermoplastic polyester carbonates. They preferably have average molecular weights ⁇ overscore (M) ⁇ w of 18,000 to 40,000 g/mol, preferably of 20,000 to 36,000 g/mol and especially of 22,000 to 35,000 g/mol, determined by measuring the relative solution viscosity in dichloromethane or in mixtures of equal quantities by weight of phenol/o-dichlorobenzene calibrated by light scattering.
- M average molecular weights ⁇ overscore (M) ⁇ w of 18,000 to 40,000 g/mol, preferably of 20,000 to 36,000 g/mol and especially of 22,000 to 35,000 g/mol, determined by measuring the relative solution viscosity in dichloromethane or in mixtures of equal quantities by weight of phenol/o-dichlorobenzene calibrated by light scattering.
- the production of polycarbonate preferably takes place by the interfacial polycondensation process or the melt transesterification process and is described using the interfacial polycondensation process as an example.
- Compounds preferably to be used as starting compounds are bisphenols of the general formula HO—Z—OH, wherein Z is a divalent organic radical with 6 to 30 carbon atoms, which contains one or more aromatic groups.
- Z is a divalent organic radical with 6 to 30 carbon atoms, which contains one or more aromatic groups.
- Examples of such compounds are bisphenols belonging to the group of the dihydroxydiphenyls, bis(hydroxyphenyl)-alkanes, indane bisphenols, bis(hydroxyphenyl) ethers, bis(hydroxyphenyl) sulfones, bis(hydroxyphenyl) ketones and ⁇ , ⁇ ′-bis(hydroxyphenyl)diisopropylbenzenes.
- Preferred bisphenols belonging to the above-mentioned groups of compounds are bisphenol A, tetraalkylbisphenol A, 4,4-(meta-phenylenediisopropyl) diphenol (bisphenol M), 4,4-(para-phenylenediisopropyl) diphenol, 1,1-bis(4-hydroxyphenyl)-3,3,5-trimethylcyclohexane (BP-TMC), 2,2-bis(4-hydroxyphenyl)-2-phenylethane, 1,1-bis(4-hydroxyphenyl)cyclohexane (bisphenol Z) and optionally mixtures thereof.
- the bisphenol compounds to be used according to the invention are preferably reacted with carbonic acid compounds, especially phosgene or, in the melt transesterification process, preferably with diphenyl carbonate or dimethyl carbonate.
- Polyester carbonates are obtained by reacting the bisphenols already mentioned, at least one aromatic dicarboxylic acid and optionally carbonic acid equivalents.
- Suitable aromatic dicarboxylic acids for this purpose are, for example, phthalic acid, terephthalic acid, isophthalic acid, 3,3′- or 4,4′-diphenyldicarboxylic acid and benzophenonedicarboxylic acids.
- a part, up to 80 mole %, preferably 20 to 50 mole %, of the carbonate groups in the polycarbonates can be replaced by aromatic dicarboxylate groups and are also referred to as polycarbonate according to the invention.
- Inert organic solvents used in the interfacial polycondensation process are, for example, dichloromethane, the various dichloroethanes and chloropropane compounds, tetra-chloromethane, trichloromethane, chlorobenzene and chlorotoluene; chlorobenzene or dichloromethane or mixtures of dichloromethane and chlorobenzene are preferably used.
- the interfacial polycondensation reaction can be accelerated by catalysts, such as tertiary amines, especially N-alkylpiperidines or onium salts.
- catalysts such as tertiary amines, especially N-alkylpiperidines or onium salts.
- Tributylamine, triethylamine and N-ethylpiperidine are preferably used.
- the catalysts mentioned in DE-A 4 238 123 are used.
- the polycarbonates can be branched in a deliberate and controlled manner by using small quantities of branching agents.
- branching agents are: phloroglucinol, 4,6-dimethyl-2,4,6-tri(4-hydroxyphenyl)-2-heptene; 4,6-dimethyl-2,4,6-tri(4-hydroxyphenyl)heptane; 1,3,5-tri(4-hydroxyphenyl)benzene; 1,1,1-tri(4-hydroxyphenyl)ethane; tri(4-hydroxyphenyl)phenylmethane; 2,2-bis[4,4-bis(4-hydroxyphenyl)cyclohexyl]propane; 2,4-bis(4-hydroxyphenylisopropyl)phenol; 2,6-bis(2-hydroxy-5′-methylbenzyl)-4-methylphenol; 2-(4-hydroxyphenyl)-2-(2,4-dihydroxyphenyl)propane; hexa(4-(4-hydroxyphenyliso
- the 0.05 to 2 mole %, based on bisphenols used, of branching agents or mixtures of branching agents that can optionally be incorporated can be fed in together with the bisphenols or added at a later stage of the synthesis.
- Chain terminators can be used.
- Phenols such as phenol, allkylphenols such as cresol and 4-tert-butylphenol, chlorophenol, bromophenol, cumylphenol or mixtures thereof are preferably used as chain terminators in quantities of preferably 1-20 mole %, particularly preferably 2-10 mole % per mole of bisphenol. Phenol, 4-tert-butylphenol and cumylphenol are preferred.
- Chain terminators and branching agents can be added separately or together with the bisphenol during the synthesis.
- the incorporation of the UV absorbers into the compositions according to the invention takes place by conventional methods, e.g. by mixing solutions of the UV absorbers with solutions of the plastics in suitable organic solvents, such as CH 2 Cl 2 , haloalkanes, halogenated aromatics, chlorobenzene and xylenes.
- suitable organic solvents such as CH 2 Cl 2 , haloalkanes, halogenated aromatics, chlorobenzene and xylenes.
- the substance mixtures are then homogenised by known means, preferably by extrusion.
- the solution mixtures are removed by known means by evaporating the solvent followed by extrusion, e.g. in extruders.
- compositions according to the invention can additionally contain suitable mould release agents.
- Suitable mould release agents are e.g. the esters of long-chain aliphatic acids and alcohols.
- Esters of fatty acid alcohols or polyols such as e.g. pentaerythritol with fatty acids, as described in DE-A 33 12 158, EP-A 0 100 918, EP-A 0 103 107, EP-A 0 561 629, EP-A 0 352 458, EP-A 0 436 117, or esters of fatty acids with Guerbet alcohols, which are described e.g. in U.S. Pat. No. 5,001,180, DE-A 33 12 157, U.S. Pat. No. 5,744,626, can be mentioned here as examples.
- compositions according to the invention can additionally contain stabilisers.
- Suitable stabilisers are, for example, phosphines, phosphites or epoxides or Si-containing stabilisers and other compounds described in EP-A 0 500 496 and U.S. Pat. No. 3,673,146.
- Triphenyl phosphites, diphenylalkyl phosphites, phenyldialkyl phosphites, tris(nonylphenyl) phosphite, tetrakis(2,4-di-tert-butylphenyl)-4,4′-biphenylene diphosphonite and triaryl phosphite can be mentioned as examples.
- Triphenyl-phosphine and tris(2,4-di-tert-butylphenyl) phosphite are particularly preferred.
- compositions according to the invention and the products made therefrom can contain organic dyes, inorganic pigments, fluorescent dyes and particularly preferably optical brighteners.
- compositions according to the invention can be used for the extrusion of sheets. These sheets can be provided on one or both sides with co-extrusion layers.
- Suitable UV absorbers for the co-extrusion moulding compositions are preferably those compounds capable of effectively protecting polycarbonate from UV light owing to their absorption capacity below 400 nm and having a molecular weight of preferably more than 370, preferably 500 and more.
- Suitable UV absorbers are especially the compounds of formula (II) described in WO 99/05205
- R 1 and R 2 are the same or different and signify H, halogen, C 1 -C 10 alky, C 5 -C 10 cycloalkyl, C 7 -C 13 aralkyl, C 6 -C 14 aryl, —OR 5 or —(CO)—O—R 5 with
- R 5 H or C 1 -C 4 alkyl
- R 3 and R 4 are also the same or different and signify H, C 1 -C 4 alkyl, C 5 -C 6 cycloalkyl, benzyl or C 6 -C 14 aryl,
- m is 1, 2 or 3 and
- n 1, 2, 3 or 4
- R 1 , R 2 , m and n have the meaning given for formula (II), and wherein
- p is an integer from 0 to 3
- q is an integer from 1 to 10
- Y is —CH 2 —CH 2 —, —(CH 2 ) 3 —, —(CH 2 ) 4 —, —(CH 2 ) 5 —, —(CH 2 ) 6 — or CH(CH 3 )—CH 2 — and
- R 3 and R 4 have the meaning given for formula (II).
- V absorbers are those which represent substituted triazines, such as 2,4-bis(2,4-dimethylphenyl)-6-(2-hydroxy-4-n-octyloxyphenyl)-1,3,5-triazine (CYASORB® UV-1 164) or 2-(4,6-diphenyl-1,3,5-triazin-2-yl)-5-(hexyl)oxyphenol (Tinuvin® 1577).
- CYASORB® UV-1 164 2-(4,6-diphenyl-1,3,5-triazin-2-yl)-5-(hexyl)oxyphenol
- Particularly preferred as a UV absorber is 2,2-methylenebis( 4-(1,1,3,3-tetramethylbutyl)-6-(2H-benzotriazol-2-yl)phenol, which is marketed commercially as Tinuvin® 360 or Adeka Stab® LA 31.
- UV absorbers mentioned in EP-A 0 500 496 are also suitable.
- the UV absorber Uvinul® 3030 from BASF AG obtained in WO 96/15102, example 1, (formula (I) with R 1 to R 40 H) can also be used.
- compositions according to the invention can additionally contain antistatic agents.
- antistatic agents are cationic compounds, e.g. quaternary ammonium, phosphonium or sulfonium salts, anionic compounds, e.g. alkyl sulfonates, alkyl sulfates, alkyl phosphates, carboxylates in the form of alkali or alkaline-earth metal salts, non-ionogenic compounds, e.g. polyethylene glycol esters, polyethylene glycol ethers, fatty acid esters, ethoxylated fatty amines.
- Preferred antistatic agents are non-ionogenic compounds.
- the mixing of the individual components can take place by known means, both successively and simultaneously and both at ambient temperature and at elevated temperature.
- the incorporation of the additives into the compositions according to the invention preferably takes place by known means by mixing polymer granules with the additives and then extruding, or by mixing the solutions of polycarbonate with solutions of the additives and then evaporating the solvents by known means.
- the proportion of additives can be varied within broad limits and depends on the desired properties of the compositions.
- the total proportion of additives in the composition is preferably 0.01 to 20 wt. %, preferably 0.05 to 5 wt. %, particularly preferably 0.1 to 1.5 wt. %, based on the weight of the composition.
- compositions thus obtained can be converted to shaped objects (products), such as e.g. parts for toys, but also fibres, films, film tapes, solid sheets, multi wall sheets, corrugated sheets, vessels, pipes and other profiles, by the conventional methods, such as e.g. hot press moulding, spinning, extruding or injection moulding.
- shaped objects such as e.g. parts for toys, but also fibres, films, film tapes, solid sheets, multi wall sheets, corrugated sheets, vessels, pipes and other profiles.
- the compositions can also be processed into cast films.
- the invention thus also relates to the use of the compositions according to the invention for the production of a shaped object (products).
- the use of multi-layer systems is also of interest.
- the invention also provides products that have been made incorporating the compositions according to the invention.
- the compositions according to the invention can be used for the production of solid plastic sheets and multi wall sheets (e.g. twin wall sheets, triple wall sheets etc.).
- the sheets also include those having an additional outer layer with an increased content of UV absorbers on one side or on both sides.
- compositions according to the invention enable products to be made with improved optical properties, especially sheets and products made from them, such as e.g. glazing (solid sheets, multi wall sheets and corrugated sheets) and structural parts in the building sector and in the automotive sector, and also plastic headlight lenses and spectacle lenses and lamp housings, lamp covers, housings for the electronics industry, bottles and films.
- sheets and products made from them such as e.g. glazing (solid sheets, multi wall sheets and corrugated sheets) and structural parts in the building sector and in the automotive sector, and also plastic headlight lenses and spectacle lenses and lamp housings, lamp covers, housings for the electronics industry, bottles and films.
- thermoforming or surface treatments e.g. coating with scratch resistant paints, water-spreading coatings, vapour deposition, sputtering, laminating with films or sheets and similar, are possible and the products made by these processes are also provided by the patent.
Abstract
A transparent thermoplastic molding composition suitable for the preparation of UV-stable products having high light transmission and low haze values is disclosed. The composition contains a transparent thermoplastic polymer and one or more compounds conforming to formula (I)
wherein R1 to R40 independently denote a member selected from the group consisting of H, alkyl, halogen and —CN.
Description
-
- wherein
- R 1 to R40 are the same or different and are selected from the group consisting of H, alkyl, halogen and —CN,
- and products made therefrom.
- Polycarbonate sheets are known, e.g. from EP-A 0 110 221, and are provided for a large number of applications. Production takes place e.g. by extrusion of compositions containing polycarbonate and optionally co-extrusion with compositions containing polycarbonate that can contain an elevated proportion of UV absorbers.
- Products of compositions containing polycarbonate, such as e.g. spectacle lenses and plastic headlight lenses, are preferably made by injection moulding. Automobile glazing can be made by either injection moulding or extrusion, as desired.
- Important factors in the selection of transparent polycarbonate sheets are high light transmission and low haze. So that the UV portion of sunlight does not lead to severe yellowing of the sheets, the polycarbonate normally incorporates at least one UV stabiliser. In the case of transparent glazing, increased haze of the sheet leads to poorer visibility of objects behind it. The haze effect is optically more noticeable the thicker the sheet. Low haze is particularly important, for example, in automobile glazing.
- DE-A 1 670 951 teaches that UV absorbers based, e.g., on substituted benzotriazoles are used for stabilising polycarbonate against yellowing under the action of UV light. For long-term protection from yellowing by UV light, EP-A 0 320 632 teaches that the polycarbonate sheets should incorporate a co-extrusion layer containing low-volatility UV absorbers, especially dimeric benzotriazoles, such as e.g. Tinuvin® 360 (bis[2-hydroxy-5-tert-octyl-3-(benzotriazol-2-yl)phenyl]methane), a product of Ciba Spezialitätenchemie, Basel, Switzerland, in a sufficiently high concentration.
- WO 96/15102 describes special UV absorbers, including those according to formula (I), which act as a light stabiliser or a stabiliser for organic materials, such as e.g. plastics.
- WO 96/15102 teaches that a content of 0.01 to 10 wt. % of the UV absorbers described there bring about UV protection in organic materials. However, nothing is said about the quality of the UV protection in polycarbonate. Also, no teaching is disclosed as to how low-haze compositions can be obtained.
- The present invention is based on the object of providing compositions that are stable towards UV radiation and exhibit high light transmission and low haze. Furthermore, products made from these compositions are to be provided.
- The object according to the invention is achieved by compositions containing
- a) a transparent thermoplastic polymer and
-
- wherein
- R 1 to R40 are the same or different and are selected from the group consisting of H, alkyl, halogen and —CN,
- and products made therefrom.
- The composition according to the invention preferably contains 0.08 to 0.6 wt. %, particularly preferably 0.1 to 0.3 wt. %, of the compounds according to formula (I). R 1 to R40 preferably equal H (hydrogen).
- The transparent thermoplastic polymer according to the invention is preferably selected from the group consisting of polycarbonate, polymethyl methacrylate, polyethyl methacrylate, polystyrene, polysulfone, styrene-acrylonitrile copolymer, polyester, polyethylene terephthalate, polybutylene terephthalate, copolyesters of polyethylene terephthalate with cyclohexanedimethanol, copolyesters of polybutylene terephthalate with cyclohexanedimethanol, polyether sulfone, polyethylene, polypropylene and mixtures of the above polymers, those polymers and mixtures that can be processed into highly transparent, crystal clear products being preferred.
- The transparent thermoplastic polymer polycarbonate is especially preferred.
- Polycarbonates preferred according to the invention are polycarbonates based on bisphenol A, especially bisphenol A homopolycarbonate and copolycarbonates based on bisphenol A and 1,1-bis(4-hydroxyphenyl)-3,3,5-trimethylcyclohexane.
- Particularly suitable transparent thermoplastic polymers are also copolycarbonates based on bisphenols, poly- or copolyacrylates and poly- or copolymethacrylates, such as e.g. poly- or copolymethyl methacrylate, copolymers with styrene, such as e.g. transparent polystyrene-acrylonitrile (SAN), and also transparent cycloolefins, poly- or copolycondensates of terephthalic acid, such as e.g. poly- or copolyethylene terephthalate (PET or CoPET or PETG).
- Examples of polyesters and copolyesters are described in EP-A 0 678 376, EP-A 0 595 413 and U.S. Pat. No. 6,096,854.
- Further, the compositions according to the invention preferably also contain 0.01 to 1 wt. %, particularly preferably 0.04 to 0.7 wt. %, pentaerythritol tetrastearate or glycerol monostearate or fatty acid esters of Guerbet alcohols or mixtures thereof.
- The object according to the invention is also achieved by products containing the composition according to the above paragraphs.
- Products selected from the group consisting of sheets, solid sheets, multi wall sheets, corrugated sheets, glazing panels, greenhouses, conservatories, bus shelters, advertising panels, signs, safety screens, automobile glazing, windows, roofing, plastic headlight lenses and spectacle lenses are preferred.
- Products that are multi-layer, and in which at least one layer contains a sufficiently high content of a UV absorber that the layers below it are protected from the harmful effects of UV light, are also preferred. A preferred embodiment of the present invention is provided by the fact that the UV absorber contained in at least one layer is a compound according to formula (I). Another preferred embodiment of the present invention is provided by the fact that the UV absorber contained in at least one layer is another compound.
- Another preferred embodiment according to the invention is provided by the fact that the transparent thermoplastic polymer is a polymer blend consisting of at least 20 wt. % polycarbonate, the polymer blend also containing polyesters or polymethacrylates or both as blend partners in addition to polycarbonate.
- The compound according to formula (I) with R 1 to R40 equal to H is commercially available as Uvinul® 3030 from BASF AG, Ludwigshafen, Germany.
- The production of the compound according to formula (I) with R 1 to R40 equal to H is described in WO 96/15102. The compounds according to formula a) in which R1 to R40 have a different meaning can be produced by corresponding processes.
- Since the compounds according to formula (I) are of low volatility, it is possible to use them as described in EP-A 0 320 632 in compositions containing transparent thermoplastic polymers as UV protection for co-extruded sheets (use in co-extrusion moulding compositions).
- Surprisingly, it has been shown that, when the compounds according to formula (I) are used in concentrations of 0.08 to 0.6 wt. %, preferably 0.1 to 0.3 wt. %, the haze and light transmission are better than with the conventional benzotriazole UV absorbers.
- The polycarbonates according to the invention are homopolycarbonates, copolycarbonates and thermoplastic polyester carbonates. They preferably have average molecular weights {overscore (M)} w of 18,000 to 40,000 g/mol, preferably of 20,000 to 36,000 g/mol and especially of 22,000 to 35,000 g/mol, determined by measuring the relative solution viscosity in dichloromethane or in mixtures of equal quantities by weight of phenol/o-dichlorobenzene calibrated by light scattering.
- For the production of polycarbonates for the compositions according to the invention, reference is made, for example, to Schnell, “Chemistry and Physics of Polycarbonates”, Polymer Reviews, vol. 9, Interscience Publishers, New York, London, Sydney 1964, to D. C. PREVORSEK, B. T. DEBONA and Y. KESTEN, Corporate Research Center, Allied Chemical Corporation, Moristown, N.J. 07960, “Synthesis of Poly(ester)carbonate Copolymers” in Journal of Polymer Science, Polymer Chemistry Edition, vol. 19, 75-90 (1980), to D. Freitag, U. Grigo, P. R. Müller, N. Nouvertne, BAYER AG, “Polycarbonates” in Encyclopedia of Polymer Science and Engineering, vol. 11, second edition, 1988, pages 648-718 and finally to Drs. U. Grigo, K. Kircher and P. R Müller “Polycarbonate” in Becker/Braun, Kunststoff-Handbuch, vol. 3/1, Polycarbonate, Polyacetale, Polyester, Celluloseester, Carl Hanser Verlag Munich, Vienna, 1992, pages 117-299.
- The production of polycarbonate preferably takes place by the interfacial polycondensation process or the melt transesterification process and is described using the interfacial polycondensation process as an example.
- Compounds preferably to be used as starting compounds are bisphenols of the general formula HO—Z—OH, wherein Z is a divalent organic radical with 6 to 30 carbon atoms, which contains one or more aromatic groups. Examples of such compounds are bisphenols belonging to the group of the dihydroxydiphenyls, bis(hydroxyphenyl)-alkanes, indane bisphenols, bis(hydroxyphenyl) ethers, bis(hydroxyphenyl) sulfones, bis(hydroxyphenyl) ketones and α,α′-bis(hydroxyphenyl)diisopropylbenzenes.
- Preferred bisphenols belonging to the above-mentioned groups of compounds are bisphenol A, tetraalkylbisphenol A, 4,4-(meta-phenylenediisopropyl) diphenol (bisphenol M), 4,4-(para-phenylenediisopropyl) diphenol, 1,1-bis(4-hydroxyphenyl)-3,3,5-trimethylcyclohexane (BP-TMC), 2,2-bis(4-hydroxyphenyl)-2-phenylethane, 1,1-bis(4-hydroxyphenyl)cyclohexane (bisphenol Z) and optionally mixtures thereof.
- The bisphenol compounds to be used according to the invention are preferably reacted with carbonic acid compounds, especially phosgene or, in the melt transesterification process, preferably with diphenyl carbonate or dimethyl carbonate. Polyester carbonates are obtained by reacting the bisphenols already mentioned, at least one aromatic dicarboxylic acid and optionally carbonic acid equivalents. Suitable aromatic dicarboxylic acids for this purpose are, for example, phthalic acid, terephthalic acid, isophthalic acid, 3,3′- or 4,4′-diphenyldicarboxylic acid and benzophenonedicarboxylic acids. A part, up to 80 mole %, preferably 20 to 50 mole %, of the carbonate groups in the polycarbonates can be replaced by aromatic dicarboxylate groups and are also referred to as polycarbonate according to the invention.
- Inert organic solvents used in the interfacial polycondensation process are, for example, dichloromethane, the various dichloroethanes and chloropropane compounds, tetra-chloromethane, trichloromethane, chlorobenzene and chlorotoluene; chlorobenzene or dichloromethane or mixtures of dichloromethane and chlorobenzene are preferably used.
- The interfacial polycondensation reaction can be accelerated by catalysts, such as tertiary amines, especially N-alkylpiperidines or onium salts. Tributylamine, triethylamine and N-ethylpiperidine are preferably used. In the case of the melt transesterification process, e.g. the catalysts mentioned in DE-A 4 238 123 are used.
- The polycarbonates can be branched in a deliberate and controlled manner by using small quantities of branching agents. Some suitable branching agents are: phloroglucinol, 4,6-dimethyl-2,4,6-tri(4-hydroxyphenyl)-2-heptene; 4,6-dimethyl-2,4,6-tri(4-hydroxyphenyl)heptane; 1,3,5-tri(4-hydroxyphenyl)benzene; 1,1,1-tri(4-hydroxyphenyl)ethane; tri(4-hydroxyphenyl)phenylmethane; 2,2-bis[4,4-bis(4-hydroxyphenyl)cyclohexyl]propane; 2,4-bis(4-hydroxyphenylisopropyl)phenol; 2,6-bis(2-hydroxy-5′-methylbenzyl)-4-methylphenol; 2-(4-hydroxyphenyl)-2-(2,4-dihydroxyphenyl)propane; hexa(4-(4-hydroxyphenylisopropyl)phenyl) orthoterephthalate; tetra(4-hydroxyphenyl)methane; tetra(4-(4-hydroxyphenylisopropyl)phenoxy)methane; α,α′,α″-tris(4-hydroxyphenyl)-1,3,5-triisopropylbenzene; 2,4-dihydroxybenzoic acid; trimesic acid; cyanuric chloride; 3,3-bis(3-methyl-4-hydroxyphenyl )-2-oxo-2,3-dihydroindole; 1,4-bis-(4′,4″-dihydroxytriphenyl)methyl)benzene and especially: 1,1,1-tri(4-hydroxyphenyl)ethane and bis(3-methyl-4-hydroxyphenyl)-2-oxo-2,3-dihydroindole.
- The 0.05 to 2 mole %, based on bisphenols used, of branching agents or mixtures of branching agents that can optionally be incorporated can be fed in together with the bisphenols or added at a later stage of the synthesis.
- Chain terminators can be used. Phenols such as phenol, allkylphenols such as cresol and 4-tert-butylphenol, chlorophenol, bromophenol, cumylphenol or mixtures thereof are preferably used as chain terminators in quantities of preferably 1-20 mole %, particularly preferably 2-10 mole % per mole of bisphenol. Phenol, 4-tert-butylphenol and cumylphenol are preferred.
- Chain terminators and branching agents can be added separately or together with the bisphenol during the synthesis.
- The production of the polycarbonates for the compositions according to the invention by the melt transesterification process is described, for example, in DE-A 4 238 123.
- The incorporation of the UV absorbers into the compositions according to the invention takes place by conventional methods, e.g. by mixing solutions of the UV absorbers with solutions of the plastics in suitable organic solvents, such as CH 2Cl2, haloalkanes, halogenated aromatics, chlorobenzene and xylenes. The substance mixtures are then homogenised by known means, preferably by extrusion. The solution mixtures are removed by known means by evaporating the solvent followed by extrusion, e.g. in extruders.
- The compositions according to the invention can additionally contain suitable mould release agents.
- Suitable mould release agents are e.g. the esters of long-chain aliphatic acids and alcohols. Esters of fatty acid alcohols or polyols, such as e.g. pentaerythritol with fatty acids, as described in DE-A 33 12 158, EP-A 0 100 918, EP-A 0 103 107, EP-A 0 561 629, EP-A 0 352 458, EP-A 0 436 117, or esters of fatty acids with Guerbet alcohols, which are described e.g. in U.S. Pat. No. 5,001,180, DE-A 33 12 157, U.S. Pat. No. 5,744,626, can be mentioned here as examples.
- The compositions according to the invention can additionally contain stabilisers.
- Suitable stabilisers are, for example, phosphines, phosphites or epoxides or Si-containing stabilisers and other compounds described in EP-A 0 500 496 and U.S. Pat. No. 3,673,146. Triphenyl phosphites, diphenylalkyl phosphites, phenyldialkyl phosphites, tris(nonylphenyl) phosphite, tetrakis(2,4-di-tert-butylphenyl)-4,4′-biphenylene diphosphonite and triaryl phosphite can be mentioned as examples. Triphenyl-phosphine and tris(2,4-di-tert-butylphenyl) phosphite are particularly preferred.
- Furthermore, the compositions according to the invention and the products made therefrom can contain organic dyes, inorganic pigments, fluorescent dyes and particularly preferably optical brighteners.
- The compositions according to the invention can be used for the extrusion of sheets. These sheets can be provided on one or both sides with co-extrusion layers.
- Co-extrusion per se is known from the literature (cf. e.g. EP-A 0 110 221 and EP-A 0 110238).
- Suitable UV absorbers for the co-extrusion moulding compositions that can optionally be used are preferably those compounds capable of effectively protecting polycarbonate from UV light owing to their absorption capacity below 400 nm and having a molecular weight of preferably more than 370, preferably 500 and more.
-
- wherein
- R 1 and R2 are the same or different and signify H, halogen, C1-C10 alky, C5-C10 cycloalkyl, C7-C13 aralkyl, C6-C14 aryl, —OR5 or —(CO)—O—R5 with
- R 5=H or C1-C4 alkyl,
- R 3and R4 are also the same or different and signify H, C1-C4 alkyl, C5-C6 cycloalkyl, benzyl or C6-C14 aryl,
- m is 1, 2 or 3 and
- n is 1, 2, 3 or 4,
-
-
- and
- R 1, R2, m and n have the meaning given for formula (II), and wherein
- p is an integer from 0 to 3,
- q is an integer from 1 to 10,
- Y is —CH 2—CH2—, —(CH2)3—, —(CH2)4—, —(CH2)5—, —(CH2)6— or CH(CH3)—CH2— and
- R 3 and R4 have the meaning given for formula (II).
- Other suitable V absorbers are those which represent substituted triazines, such as 2,4-bis(2,4-dimethylphenyl)-6-(2-hydroxy-4-n-octyloxyphenyl)-1,3,5-triazine (CYASORB® UV-1 164) or 2-(4,6-diphenyl-1,3,5-triazin-2-yl)-5-(hexyl)oxyphenol (Tinuvin® 1577). Particularly preferred as a UV absorber is 2,2-methylenebis( 4-(1,1,3,3-tetramethylbutyl)-6-(2H-benzotriazol-2-yl)phenol, which is marketed commercially as Tinuvin® 360 or Adeka Stab® LA 31. The UV absorbers mentioned in EP-A 0 500 496 are also suitable. The UV absorber Uvinul® 3030 from BASF AG obtained in WO 96/15102, example 1, (formula (I) with R 1 to R40=H) can also be used.
- The compositions according to the invention can additionally contain antistatic agents.
- Examples of antistatic agents are cationic compounds, e.g. quaternary ammonium, phosphonium or sulfonium salts, anionic compounds, e.g. alkyl sulfonates, alkyl sulfates, alkyl phosphates, carboxylates in the form of alkali or alkaline-earth metal salts, non-ionogenic compounds, e.g. polyethylene glycol esters, polyethylene glycol ethers, fatty acid esters, ethoxylated fatty amines. Preferred antistatic agents are non-ionogenic compounds.
- All feedstock and solvents used for the synthesis of the compositions according to the invention can be contaminated with corresponding impurities from their production and storage, the aim being to work with the cleanest starting substances possible.
- The mixing of the individual components can take place by known means, both successively and simultaneously and both at ambient temperature and at elevated temperature.
- The incorporation of the additives into the compositions according to the invention preferably takes place by known means by mixing polymer granules with the additives and then extruding, or by mixing the solutions of polycarbonate with solutions of the additives and then evaporating the solvents by known means.
- The proportion of additives can be varied within broad limits and depends on the desired properties of the compositions.
- The total proportion of additives in the composition is preferably 0.01 to 20 wt. %, preferably 0.05 to 5 wt. %, particularly preferably 0.1 to 1.5 wt. %, based on the weight of the composition.
- The compositions thus obtained can be converted to shaped objects (products), such as e.g. parts for toys, but also fibres, films, film tapes, solid sheets, multi wall sheets, corrugated sheets, vessels, pipes and other profiles, by the conventional methods, such as e.g. hot press moulding, spinning, extruding or injection moulding. The compositions can also be processed into cast films.
- The invention thus also relates to the use of the compositions according to the invention for the production of a shaped object (products). The use of multi-layer systems is also of interest.
- The invention also provides products that have been made incorporating the compositions according to the invention. The compositions according to the invention can be used for the production of solid plastic sheets and multi wall sheets (e.g. twin wall sheets, triple wall sheets etc.). The sheets also include those having an additional outer layer with an increased content of UV absorbers on one side or on both sides.
- The compositions according to the invention enable products to be made with improved optical properties, especially sheets and products made from them, such as e.g. glazing (solid sheets, multi wall sheets and corrugated sheets) and structural parts in the building sector and in the automotive sector, and also plastic headlight lenses and spectacle lenses and lamp housings, lamp covers, housings for the electronics industry, bottles and films.
- Subsequent treatments of the products made with the compositions according to the invention, such as e.g. thermoforming or surface treatments, e.g. coating with scratch resistant paints, water-spreading coatings, vapour deposition, sputtering, laminating with films or sheets and similar, are possible and the products made by these processes are also provided by the patent.
- The invention is further illustrated by the following examples, without being limited to these.
- To prepare the test pieces for tests A to H, a polycarbonate with the trade name Makrolon® 3108 (linear bisphenol A polycarbonate from Bayer AG, Leverkusen, Germany, with a melt flow index (MFR) of 6.5 g/10 min at 300° C. and 1.2 kg load) was compounded with the specified quantity of UV absorber at 310° C. on a twin screw extruder and then granulated. Rectangular sheets (60 mm×40 mm×3 mm) were then made from these granules by injection moulding.
- The weathering of these sheets took place in a Weather-o-meter from Atlas, USA, with a 6.5 W xenon burner in a cycle of 102 min illumination and 18 min spraying with demineralised water with illumination. The maximum black panel temperature was 60° C. (±5° C.).
- The light transmission, the yellowness index and the haze were determined in accordance with ASTM specification D 1003 using Haze-Gard plus apparatus from BYK-Gardner GmbH, D-82538 Geretsried.
- Table 1 gives a compilation of the results.
TABLE 1 No. UV absorber Haze Transmission A 0.3% Tinuvin ® 350 3.7 84.0% B 0.2% Tinuvin ® 350 1.9 85.4% C 0.1% Tinuvin ® 350 1.3 85.9% D 0.8% Uvinul ® 3030 4.2 83.7% E 0.3% Uvinul ® 3030 1.4 85.8% F 0.2% Uvinul ® 3030 1.2 86.0% G 0.1% Uvinul ® 3030 0.9 86.2% H 0.02% Uvinul ® 3030 0.5 86.5% - The result shows that the injection moulded sheets incorporating Uvinul® 3030 have lower haze values and better light transmission values than the injection moulded sheets incorporating Tinuvin® 350. This is true at least in a range of concentration of 0.1 to 0.3 wt. %.
- At concentrations of Uvinul® 3030 that are too high (0.8%), the haze becomes too great. Polycarbonate sheets with such high haze are no longer usable.
TABLE 2 Development of the yellowness index and haze with artificial weathering (Xe-WOM), (cycles: 102 min irradiation; 18 min irradiation + water spray) Yellowness index No. 0 h 5000 h 8000 h B 4.9 17.3 22.4 F 4.9 16.4 21.5 H 4.1 21.4 29.3 - The result shows that B and F are very similar in their weathering behaviour. H is striking for its excessively marked yellowing. These polycarbonate sheets are not suitable for glazing. The concentration of 0.02 wt. % Uvinul® 3030 is too low for adequate UV stabilising.
Claims (11)
2. Composition according to claim 1 , wherein R1 to R40 are equal to H.
3. Composition according to claims 1 or 2, which contain 0.08 to 0.6 wt. % of the compounds according to formula (I).
4. Composition according to any one of claims 1 to 3 , wherein the transparent thermoplastic polymer is selected from the group consisting of polycarbonate, polymethyl methacrylate, polyethyl methacrylate, polystyrene, polysulfone, styrene-acrylonitrile copolymer, polyester, polyethylene terephthalate, polybutylene terephthalate, copolyesters of polyethylene terephthalate with cyclohexanedimethanol, copolyesters of polybutylene terephthalate with cyclohexanedimethanol, polyether sulfone, polyethylene, polypropylene and mixtures of the above polymers.
5. Composition according to any one of claims 1 to 3 , wherein the transparent thermoplastic polymer is polycarbonate.
6. Composition according to any one of claims 1 to 5 , which additionally contains 0.01 to 1 wt. % pentaerythritol tetrastearate or glycerol monostearate or fatty acid esters of Guerbet alcohols or mixtures thereof.
7. Product containing the composition according to any one of claims 1 to 6 .
8. Product according to claim 7 , wherein the product is selected from the group consisting of sheets, solid sheets, multi wall sheets, corrugated sheets, glazing panels, greenhouses, conservatories, bus shelters, advertising panels, signs, safety screens, automobile glazing, windows, roofing, plastic headlight lenses and spectacle lenses.
9. Product according to claim 7 or 8, wherein the product is multi-layer and wherein at least one layer contains a sufficiently high content of a UV absorber that the layers below it are protected from the harmful effects of UV light.
10. Product according to claim 9 , wherein the layer with the increased proportion of UV absorber is applied by co-extrusion.
11. Product according to claim 9 , wherein the layer with the increased proportion of UV absorber is applied by painting.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE10026628.2 | 2000-05-29 | ||
| DE10026628A DE10026628A1 (en) | 2000-05-29 | 2000-05-29 | Transparent thermoplastic compositions |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030130390A1 true US20030130390A1 (en) | 2003-07-10 |
Family
ID=7643994
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/296,340 Abandoned US20030130390A1 (en) | 2000-05-29 | 2001-05-16 | Transparent thermoplastischic composition |
Country Status (16)
| Country | Link |
|---|---|
| US (1) | US20030130390A1 (en) |
| EP (1) | EP1290078B1 (en) |
| JP (1) | JP2003535169A (en) |
| KR (1) | KR100709643B1 (en) |
| CN (1) | CN1211423C (en) |
| AT (1) | ATE272678T1 (en) |
| AU (2) | AU2001265972B2 (en) |
| BR (1) | BR0111121A (en) |
| CA (1) | CA2410319C (en) |
| DE (2) | DE10026628A1 (en) |
| ES (1) | ES2225559T3 (en) |
| IL (2) | IL152911A0 (en) |
| MX (1) | MXPA02011799A (en) |
| RU (1) | RU2002135904A (en) |
| TW (1) | TWI301139B (en) |
| WO (1) | WO2001092395A1 (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040166342A1 (en) * | 2003-02-25 | 2004-08-26 | Degussa Ag | Transparent molding composition for optical applications |
| US20040166072A1 (en) * | 2002-09-06 | 2004-08-26 | The C.P. Hall Company | Photostabilization of a sunscreen composition with a combination of an alpha-cyano-beta, beta-diphenylacrylate compound and a dialkyl naphithamate |
| US6919473B2 (en) | 2002-09-17 | 2005-07-19 | Cph Innovations Corporation | Photostabilizers, UV absorbers, and methods of photostabilizing a sunscreen composition |
| US20050186154A1 (en) * | 2004-02-25 | 2005-08-25 | Bonda Craig A. | Compounds derived from polyanhydride resins with film-forming, UV-absorbing, and photostabilizing properties, compositions containing same, and methods of using the same |
| US20050222307A1 (en) * | 2002-11-22 | 2005-10-06 | Cph Innovations Corp. | Method of decreasing the UV light degradation of polymers |
| WO2006013071A1 (en) * | 2004-07-30 | 2006-02-09 | Basf Aktiengesellschaft | Stabiliser composition made from liquid and solid uv-absorbers |
| US7235587B2 (en) | 2004-07-01 | 2007-06-26 | Cph Innovations Corporation | Diesters containing two crylene or fluorene moieties, sunscreen compositions containing the same, and methods of photostabilizing a sunscreen compositions containing the same |
| US8158678B2 (en) | 2005-04-07 | 2012-04-17 | Cph Innovations Corp. | Photoabsorbing, highly conjugated compounds of cyanoacrylic esters, sunscreen compositions and methods of use |
| US8691915B2 (en) | 2012-04-23 | 2014-04-08 | Sabic Innovative Plastics Ip B.V. | Copolymers and polymer blends having improved refractive indices |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE10058290A1 (en) * | 2000-11-23 | 2002-05-29 | Basf Ag | Process for stabilizing polyolefins |
| DE10159373A1 (en) * | 2001-12-04 | 2003-06-12 | Bayer Ag | Multi-layer product |
| US6811841B1 (en) | 2003-04-15 | 2004-11-02 | 3M Innovative Properties Company | Light-stable structures |
| JP2005264132A (en) * | 2004-02-17 | 2005-09-29 | Teijin Chem Ltd | Polycarbonate resin composition |
| CN111117201B (en) * | 2020-02-25 | 2021-11-05 | 潮州明园新材料有限公司 | High-hardness PC (polycarbonate) photodiffusion material and preparation method thereof |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5108835A (en) * | 1987-11-24 | 1992-04-28 | Bayer Aktiengesellschaft | Coextruded double walled sheet of linear polycarbonate resin |
| US5821380A (en) * | 1994-11-10 | 1998-10-13 | Basf Aktiengesellschaft | 2-cyanoacrylic esters |
| US6441071B1 (en) * | 1999-09-01 | 2002-08-27 | Dow Global Technologies Inc. | Polycarbonate resin compositions comprising cyanacrylic acid ester stabilizer compounds |
| US6753367B2 (en) * | 2001-08-20 | 2004-06-22 | General Electric Company | Flame retardant polycarbonate compositions with improved weathering performance containing cyanoacrylic esters |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1553918A (en) * | 1975-09-22 | 1979-10-10 | American Cyanamid Co | Aminonitrilles and their use as stabilizers for polycarbonates |
| JPH05125328A (en) * | 1990-12-17 | 1993-05-21 | Kaken Tec Kk | Top-coating agent |
| JP2000014255A (en) * | 1998-06-30 | 2000-01-18 | Mitsui Chemicals Inc | Coating material for plant growth control |
| JP2000129112A (en) * | 1998-10-28 | 2000-05-09 | Teijin Ltd | Stabilized polycarbonate resin composition and formed product |
| JP3845213B2 (en) * | 1998-11-05 | 2006-11-15 | 帝人化成株式会社 | Polycarbonate resin composition for sheet |
| DE10006651A1 (en) * | 2000-02-15 | 2001-08-16 | Bayer Ag | Thermoplastic composition for pearly-lustre products, e.g. decorative panelling or glazing, contains pigment with a transparent core coated with three layers of metal oxide with high, low and high refractive indices respectively |
-
2000
- 2000-05-29 DE DE10026628A patent/DE10026628A1/en not_active Withdrawn
-
2001
- 2001-05-16 CA CA002410319A patent/CA2410319C/en not_active Expired - Fee Related
- 2001-05-16 AU AU2001265972A patent/AU2001265972B2/en not_active Ceased
- 2001-05-16 ES ES01943379T patent/ES2225559T3/en not_active Expired - Lifetime
- 2001-05-16 WO PCT/EP2001/005562 patent/WO2001092395A1/en active IP Right Grant
- 2001-05-16 IL IL15291101A patent/IL152911A0/en active IP Right Grant
- 2001-05-16 KR KR1020027016147A patent/KR100709643B1/en not_active IP Right Cessation
- 2001-05-16 MX MXPA02011799A patent/MXPA02011799A/en active IP Right Grant
- 2001-05-16 CN CNB018097529A patent/CN1211423C/en not_active Expired - Fee Related
- 2001-05-16 AU AU6597201A patent/AU6597201A/en active Pending
- 2001-05-16 EP EP01943379A patent/EP1290078B1/en not_active Expired - Lifetime
- 2001-05-16 DE DE50103131T patent/DE50103131D1/en not_active Expired - Lifetime
- 2001-05-16 AT AT01943379T patent/ATE272678T1/en not_active IP Right Cessation
- 2001-05-16 JP JP2002500598A patent/JP2003535169A/en active Pending
- 2001-05-16 US US10/296,340 patent/US20030130390A1/en not_active Abandoned
- 2001-05-16 RU RU2002135904/04A patent/RU2002135904A/en not_active Application Discontinuation
- 2001-05-16 BR BR0111121-3A patent/BR0111121A/en not_active IP Right Cessation
- 2001-05-22 TW TW090112168A patent/TWI301139B/en not_active IP Right Cessation
-
2002
- 2002-11-18 IL IL152911A patent/IL152911A/en not_active IP Right Cessation
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5108835A (en) * | 1987-11-24 | 1992-04-28 | Bayer Aktiengesellschaft | Coextruded double walled sheet of linear polycarbonate resin |
| US5821380A (en) * | 1994-11-10 | 1998-10-13 | Basf Aktiengesellschaft | 2-cyanoacrylic esters |
| US6441071B1 (en) * | 1999-09-01 | 2002-08-27 | Dow Global Technologies Inc. | Polycarbonate resin compositions comprising cyanacrylic acid ester stabilizer compounds |
| US6753367B2 (en) * | 2001-08-20 | 2004-06-22 | General Electric Company | Flame retardant polycarbonate compositions with improved weathering performance containing cyanoacrylic esters |
Cited By (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040166072A1 (en) * | 2002-09-06 | 2004-08-26 | The C.P. Hall Company | Photostabilization of a sunscreen composition with a combination of an alpha-cyano-beta, beta-diphenylacrylate compound and a dialkyl naphithamate |
| US6899866B2 (en) | 2002-09-06 | 2005-05-31 | Cph Innovations Corporation | Photostabilization of a sunscreen composition with a combination of an α-cyano-β, β-diphenylacrylate compound and a dialkyl naphithalate |
| US6919473B2 (en) | 2002-09-17 | 2005-07-19 | Cph Innovations Corporation | Photostabilizers, UV absorbers, and methods of photostabilizing a sunscreen composition |
| US20050222307A1 (en) * | 2002-11-22 | 2005-10-06 | Cph Innovations Corp. | Method of decreasing the UV light degradation of polymers |
| US7544350B2 (en) | 2002-11-22 | 2009-06-09 | Hallstar Innovations Corp. | Method of decreasing the UV light degradation of polymers |
| US20040166342A1 (en) * | 2003-02-25 | 2004-08-26 | Degussa Ag | Transparent molding composition for optical applications |
| US7133209B2 (en) | 2003-02-25 | 2006-11-07 | Degussa Ag | Transparent molding composition for optical applications |
| US20050186154A1 (en) * | 2004-02-25 | 2005-08-25 | Bonda Craig A. | Compounds derived from polyanhydride resins with film-forming, UV-absorbing, and photostabilizing properties, compositions containing same, and methods of using the same |
| US20050191249A1 (en) * | 2004-02-25 | 2005-09-01 | Bonda Craig A. | Compounds derived from polyanhydride resins with film-forming, UV-absorbing, and photostablizing properties, compositions containing same, and methods of using the same |
| US7534420B2 (en) | 2004-02-25 | 2009-05-19 | Hallstar Innovations Corp. | Compounds derived from polyanhydride resins with film-forming, UV-absorbing, and photostablizing properties, compositions containing same, and methods of using the same |
| US20050186152A1 (en) * | 2004-02-25 | 2005-08-25 | Bonda Craig A. | Compounds derived from polyanhydride resins with film-forming, UV-absorbing, and photostabilizing properties, compositions containing same, and methods of using the same |
| US7550134B2 (en) | 2004-02-25 | 2009-06-23 | Hallstar Innovations Corp. | Compounds derived from polyanhydride resins with film-forming, UV-absorbing, and photostabilizing properties, compositions containing same, and methods of using the same |
| US7560098B2 (en) | 2004-02-25 | 2009-07-14 | Hallstar Innovations Corp. | Compounds derived from polyanhydride resins with film-forming, UV-absorbing, and photostabilizing properties, compositions containing same, and methods of using the same |
| US7648697B2 (en) | 2004-02-25 | 2010-01-19 | Hallstar Innovations Corp. | Compounds derived from polyanhydride resins with film-forming, UV-absorbing, and photostabilizing properties, compositions containing same, and methods of using the same |
| US7235587B2 (en) | 2004-07-01 | 2007-06-26 | Cph Innovations Corporation | Diesters containing two crylene or fluorene moieties, sunscreen compositions containing the same, and methods of photostabilizing a sunscreen compositions containing the same |
| WO2006013071A1 (en) * | 2004-07-30 | 2006-02-09 | Basf Aktiengesellschaft | Stabiliser composition made from liquid and solid uv-absorbers |
| US20090264566A1 (en) * | 2004-07-30 | 2009-10-22 | Basf Aktiengesellschaft | Stabiliser composition made from liquid and solid uv-absorbers |
| US8158678B2 (en) | 2005-04-07 | 2012-04-17 | Cph Innovations Corp. | Photoabsorbing, highly conjugated compounds of cyanoacrylic esters, sunscreen compositions and methods of use |
| US8691915B2 (en) | 2012-04-23 | 2014-04-08 | Sabic Innovative Plastics Ip B.V. | Copolymers and polymer blends having improved refractive indices |
Also Published As
| Publication number | Publication date |
|---|---|
| KR100709643B1 (en) | 2007-04-23 |
| CA2410319C (en) | 2010-01-12 |
| AU2001265972B2 (en) | 2005-02-24 |
| RU2002135904A (en) | 2004-09-10 |
| EP1290078B1 (en) | 2004-08-04 |
| AU6597201A (en) | 2001-12-11 |
| JP2003535169A (en) | 2003-11-25 |
| ATE272678T1 (en) | 2004-08-15 |
| DE10026628A1 (en) | 2001-12-06 |
| KR20030005420A (en) | 2003-01-17 |
| MXPA02011799A (en) | 2003-05-14 |
| BR0111121A (en) | 2003-04-08 |
| IL152911A0 (en) | 2003-06-24 |
| EP1290078A1 (en) | 2003-03-12 |
| IL152911A (en) | 2007-10-31 |
| CA2410319A1 (en) | 2001-12-06 |
| DE50103131D1 (en) | 2004-09-09 |
| TWI301139B (en) | 2008-09-21 |
| CN1430642A (en) | 2003-07-16 |
| CN1211423C (en) | 2005-07-20 |
| ES2225559T3 (en) | 2005-03-16 |
| WO2001092395A1 (en) | 2001-12-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR100772803B1 (en) | Compositions Containing Polycarbonate and Pigments | |
| US6680350B1 (en) | Molding materials | |
| US7652082B2 (en) | Compositions containing polycarbonate and novel UV absorbers | |
| US20040013882A1 (en) | Multi-layer product containing polycarbonate | |
| AU2001265972B2 (en) | Transparent thermoplastic composition | |
| US6893689B2 (en) | Heat-absorbent multi-layer structure | |
| US20030152775A1 (en) | Multilayered article of manufacture | |
| AU2001250390B2 (en) | Compositions containing polycarbonate | |
| ES2266270T3 (en) | COMPOSITION CONTAINING THERMOPLASTIC PLASTICS. | |
| KR20020094918A (en) | Heat-absorbing polymer composition | |
| MXPA02004648A (en) | Polycarbonate moulding compounds. | |
| US6960623B2 (en) | Compositions containing polycarbonate | |
| US20050013970A1 (en) | Multi-layer product in which the co-extruded side is easily recognizable | |
| US6713181B2 (en) | Compositions containing polycarbonate | |
| ES2301570T3 (en) | COMPOSITION CONTAINING THERMOPLASTIC SYNTHETIC MATERIALS. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: BAYER AKTIENGESELLSCHAFT, GERMANY Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:GORNY, RUDIGER;ANDERS, SIEGFRIED;NISING, WOLFGANG;AND OTHERS;REEL/FRAME:013834/0129;SIGNING DATES FROM 20021002 TO 20021104 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |