US6262293B1 - ω-Cycloalkly-prostaglandin e2 derivatives - Google Patents
ω-Cycloalkly-prostaglandin e2 derivatives Download PDFInfo
- Publication number
- US6262293B1 US6262293B1 US09/582,348 US58234800A US6262293B1 US 6262293 B1 US6262293 B1 US 6262293B1 US 58234800 A US58234800 A US 58234800A US 6262293 B1 US6262293 B1 US 6262293B1
- Authority
- US
- United States
- Prior art keywords
- phenylene
- propano
- dihydroxy
- formula
- compound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 150000001875 compounds Chemical class 0.000 claims abstract description 156
- 150000003839 salts Chemical class 0.000 claims abstract description 12
- 229920000858 Cyclodextrin Polymers 0.000 claims abstract description 10
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 claims abstract description 8
- 231100000252 nontoxic Toxicity 0.000 claims abstract description 6
- 230000003000 nontoxic effect Effects 0.000 claims abstract description 6
- 239000002253 acid Substances 0.000 claims description 66
- 150000004702 methyl esters Chemical class 0.000 claims description 42
- 238000000034 method Methods 0.000 claims description 27
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 16
- 230000002378 acidificating effect Effects 0.000 claims description 15
- 125000005843 halogen group Chemical group 0.000 claims description 14
- 238000002360 preparation method Methods 0.000 claims description 12
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 10
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 claims description 9
- 125000002947 alkylene group Chemical group 0.000 claims description 8
- 125000004043 oxo group Chemical group O=* 0.000 claims description 8
- 125000006239 protecting group Chemical group 0.000 claims description 8
- 230000007062 hydrolysis Effects 0.000 claims description 7
- 238000006460 hydrolysis reaction Methods 0.000 claims description 7
- 125000004450 alkenylene group Chemical group 0.000 claims description 6
- 238000010511 deprotection reaction Methods 0.000 claims description 5
- 229910052739 hydrogen Inorganic materials 0.000 claims description 5
- 239000001257 hydrogen Substances 0.000 claims description 5
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 5
- 108090000790 Enzymes Proteins 0.000 claims description 4
- 102000004190 Enzymes Human genes 0.000 claims description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 4
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims description 3
- 238000010934 O-alkylation reaction Methods 0.000 claims description 3
- 230000009435 amidation Effects 0.000 claims description 3
- 238000007112 amidation reaction Methods 0.000 claims description 3
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 claims 4
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 claims 1
- 208000023275 Autoimmune disease Diseases 0.000 abstract description 3
- 208000010412 Glaucoma Diseases 0.000 abstract description 3
- 208000005107 Premature Birth Diseases 0.000 abstract description 3
- 206010036590 Premature baby Diseases 0.000 abstract description 3
- 206010000210 abortion Diseases 0.000 abstract description 3
- 231100000176 abortion Toxicity 0.000 abstract description 3
- 208000006673 asthma Diseases 0.000 abstract description 3
- 208000026278 immune system disease Diseases 0.000 abstract description 3
- 230000016273 neuron death Effects 0.000 abstract description 3
- 201000001119 neuropathy Diseases 0.000 abstract description 3
- 230000007823 neuropathy Effects 0.000 abstract description 3
- 210000000056 organ Anatomy 0.000 abstract description 3
- 230000011164 ossification Effects 0.000 abstract description 3
- 208000033808 peripheral neuropathy Diseases 0.000 abstract description 3
- 230000002265 prevention Effects 0.000 abstract description 3
- 239000000651 prodrug Substances 0.000 abstract description 3
- 229940002612 prodrug Drugs 0.000 abstract description 3
- 210000001525 retina Anatomy 0.000 abstract description 3
- 238000002054 transplantation Methods 0.000 abstract description 3
- 206010067125 Liver injury Diseases 0.000 abstract 1
- 231100000234 hepatic damage Toxicity 0.000 abstract 1
- 230000008818 liver damage Effects 0.000 abstract 1
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 258
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 212
- HEDRZPFGACZZDS-MICDWDOJSA-N Trichloro(2H)methane Chemical compound [2H]C(Cl)(Cl)Cl HEDRZPFGACZZDS-MICDWDOJSA-N 0.000 description 120
- 239000000203 mixture Substances 0.000 description 76
- 238000004809 thin layer chromatography Methods 0.000 description 66
- 0 C~C1C[C@@H](C)[C@H](C~CCC(O)C2(C)CCC2)C~1C*C(C)=O Chemical compound C~C1C[C@@H](C)[C@H](C~CCC(O)C2(C)CCC2)C~1C*C(C)=O 0.000 description 56
- QQONPFPTGQHPMA-UHFFFAOYSA-N C=CC Chemical compound C=CC QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 description 40
- VXNZUUAINFGPBY-UHFFFAOYSA-N C=CCC Chemical compound C=CCC VXNZUUAINFGPBY-UHFFFAOYSA-N 0.000 description 40
- YWAKXRMUMFPDSH-UHFFFAOYSA-N C=CCCC Chemical compound C=CCCC YWAKXRMUMFPDSH-UHFFFAOYSA-N 0.000 description 40
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N CCC Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 40
- QWTDNUCVQCZILF-UHFFFAOYSA-N CCC(C)C Chemical compound CCC(C)C QWTDNUCVQCZILF-UHFFFAOYSA-N 0.000 description 40
- FOTXAJDDGPYIFU-UHFFFAOYSA-N CCC1CC1 Chemical compound CCC1CC1 FOTXAJDDGPYIFU-UHFFFAOYSA-N 0.000 description 40
- IJDNQMDRQITEOD-UHFFFAOYSA-N CCCC Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 40
- VFWCMGCRMGJXDK-UHFFFAOYSA-N CCCCCl Chemical compound CCCCCl VFWCMGCRMGJXDK-UHFFFAOYSA-N 0.000 description 40
- FCBJLBCGHCTPAQ-UHFFFAOYSA-N CCCCF Chemical compound CCCCF FCBJLBCGHCTPAQ-UHFFFAOYSA-N 0.000 description 40
- CXBDYQVECUFKRK-UHFFFAOYSA-N CCCCOC Chemical compound CCCCOC CXBDYQVECUFKRK-UHFFFAOYSA-N 0.000 description 40
- 239000000243 solution Substances 0.000 description 36
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 33
- 238000006243 chemical reaction Methods 0.000 description 29
- 239000007864 aqueous solution Substances 0.000 description 25
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 24
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 22
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 20
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 19
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 19
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 19
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 18
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 18
- 239000012044 organic layer Substances 0.000 description 18
- 229920006395 saturated elastomer Polymers 0.000 description 17
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 17
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 16
- -1 methoxy, ethoxy, propoxy, butoxy Chemical group 0.000 description 16
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 15
- 238000004440 column chromatography Methods 0.000 description 15
- 239000000741 silica gel Substances 0.000 description 15
- 229910002027 silica gel Inorganic materials 0.000 description 15
- 102000005962 receptors Human genes 0.000 description 14
- 108020003175 receptors Proteins 0.000 description 14
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical group C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 13
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 12
- 229910052786 argon Inorganic materials 0.000 description 11
- 239000012298 atmosphere Substances 0.000 description 11
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 10
- 239000011780 sodium chloride Substances 0.000 description 10
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 9
- 239000002904 solvent Substances 0.000 description 9
- KRHYYFGTRYWZRS-UHFFFAOYSA-N Fluorane Chemical compound F KRHYYFGTRYWZRS-UHFFFAOYSA-N 0.000 description 8
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 8
- 102100024448 Prostaglandin E2 receptor EP2 subtype Human genes 0.000 description 8
- 235000019270 ammonium chloride Nutrition 0.000 description 8
- 239000003795 chemical substances by application Substances 0.000 description 8
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 8
- 239000011541 reaction mixture Substances 0.000 description 8
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 7
- 239000000872 buffer Substances 0.000 description 7
- 238000002347 injection Methods 0.000 description 7
- 239000007924 injection Substances 0.000 description 7
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 6
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 6
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 6
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 6
- YZXBAPSDXZZRGB-DOFZRALJSA-N arachidonic acid Chemical compound CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(O)=O YZXBAPSDXZZRGB-DOFZRALJSA-N 0.000 description 6
- 125000004432 carbon atom Chemical group C* 0.000 description 6
- 239000003960 organic solvent Substances 0.000 description 6
- 239000012047 saturated solution Substances 0.000 description 6
- OZAIFHULBGXAKX-UHFFFAOYSA-N 2-(2-cyanopropan-2-yldiazenyl)-2-methylpropanenitrile Chemical compound N#CC(C)(C)N=NC(C)(C)C#N OZAIFHULBGXAKX-UHFFFAOYSA-N 0.000 description 5
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 5
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 5
- 239000012267 brine Substances 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- 238000007796 conventional method Methods 0.000 description 5
- 239000012043 crude product Substances 0.000 description 5
- XEYBRNLFEZDVAW-ARSRFYASSA-N dinoprostone Chemical compound CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O XEYBRNLFEZDVAW-ARSRFYASSA-N 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- OAYLNYINCPYISS-UHFFFAOYSA-N ethyl acetate;hexane Chemical compound CCCCCC.CCOC(C)=O OAYLNYINCPYISS-UHFFFAOYSA-N 0.000 description 5
- 239000007788 liquid Substances 0.000 description 5
- 238000007911 parenteral administration Methods 0.000 description 5
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 5
- 125000001424 substituent group Chemical group 0.000 description 5
- 125000004191 (C1-C6) alkoxy group Chemical group 0.000 description 4
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 4
- OFBQJSOFQDEBGM-UHFFFAOYSA-N Pentane Chemical compound CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 4
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 4
- 239000004480 active ingredient Substances 0.000 description 4
- 125000000753 cycloalkyl group Chemical group 0.000 description 4
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 4
- 229960004592 isopropanol Drugs 0.000 description 4
- QPJVMBTYPHYUOC-UHFFFAOYSA-N methyl benzoate Chemical compound COC(=O)C1=CC=CC=C1 QPJVMBTYPHYUOC-UHFFFAOYSA-N 0.000 description 4
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 4
- 239000008247 solid mixture Substances 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 125000004209 (C1-C8) alkyl group Chemical group 0.000 description 3
- OISVCGZHLKNMSJ-UHFFFAOYSA-N 2,6-dimethylpyridine Chemical compound CC1=CC=CC(C)=N1 OISVCGZHLKNMSJ-UHFFFAOYSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- 125000004648 C2-C8 alkenyl group Chemical group 0.000 description 3
- 125000004649 C2-C8 alkynyl group Chemical group 0.000 description 3
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 102000016984 Prostanoids receptors Human genes 0.000 description 3
- 108070000024 Prostanoids receptors Proteins 0.000 description 3
- 125000000217 alkyl group Chemical group 0.000 description 3
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 3
- 229940114079 arachidonic acid Drugs 0.000 description 3
- 235000021342 arachidonic acid Nutrition 0.000 description 3
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 239000012230 colorless oil Substances 0.000 description 3
- 238000001816 cooling Methods 0.000 description 3
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 239000002207 metabolite Substances 0.000 description 3
- 239000008108 microcrystalline cellulose Substances 0.000 description 3
- 229940016286 microcrystalline cellulose Drugs 0.000 description 3
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 3
- PSHKMPUSSFXUIA-UHFFFAOYSA-N n,n-dimethylpyridin-2-amine Chemical compound CN(C)C1=CC=CC=N1 PSHKMPUSSFXUIA-UHFFFAOYSA-N 0.000 description 3
- 239000003921 oil Substances 0.000 description 3
- 235000019198 oils Nutrition 0.000 description 3
- 239000008177 pharmaceutical agent Substances 0.000 description 3
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 3
- 235000017557 sodium bicarbonate Nutrition 0.000 description 3
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 3
- 229960001407 sodium bicarbonate Drugs 0.000 description 3
- 239000007921 spray Substances 0.000 description 3
- 239000003381 stabilizer Substances 0.000 description 3
- 125000006272 (C3-C7) cycloalkyl group Chemical group 0.000 description 2
- HRXHZRQAZJHFGL-UHFFFAOYSA-N 1-(1-propylcyclobutyl)but-3-yn-1-ol Chemical compound C#CCC(O)C1(CCC)CCC1 HRXHZRQAZJHFGL-UHFFFAOYSA-N 0.000 description 2
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide Chemical compound CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 2
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 2
- YYROPELSRYBVMQ-UHFFFAOYSA-N 4-toluenesulfonyl chloride Chemical compound CC1=CC=C(S(Cl)(=O)=O)C=C1 YYROPELSRYBVMQ-UHFFFAOYSA-N 0.000 description 2
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 2
- UXIFOPZUXOCZOY-ZJVMVJRSSA-N COC(=O)C1=CC=C(CC[C@H]2C(Cl)CC(O)[C@@H]2/C=C/CC(O)C2(CC3CC3)CCC2)C=C1 Chemical compound COC(=O)C1=CC=C(CC[C@H]2C(Cl)CC(O)[C@@H]2/C=C/CC(O)C2(CC3CC3)CCC2)C=C1 UXIFOPZUXOCZOY-ZJVMVJRSSA-N 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical compound C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 description 2
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 2
- 108090000371 Esterases Proteins 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 2
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 2
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 2
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 2
- 108090001060 Lipase Proteins 0.000 description 2
- 239000004367 Lipase Substances 0.000 description 2
- 102000004882 Lipase Human genes 0.000 description 2
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 2
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 2
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical compound C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 2
- 102000003938 Thromboxane Receptors Human genes 0.000 description 2
- 108090000300 Thromboxane Receptors Proteins 0.000 description 2
- NLJTWWJFQJZPHW-ASZAMMKQSA-N [3H]=BSOC1CC(O)[C@H](CCC2=CC=C(C(=O)OC)C=C2)[C@H]1/C=C/CC(O/[3H]=B/S)C1(CC2CC2)CCC1 Chemical compound [3H]=BSOC1CC(O)[C@H](CCC2=CC=C(C(=O)OC)C=C2)[C@H]1/C=C/CC(O/[3H]=B/S)C1(CC2CC2)CCC1 NLJTWWJFQJZPHW-ASZAMMKQSA-N 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 125000003342 alkenyl group Chemical group 0.000 description 2
- 235000003704 aspartic acid Nutrition 0.000 description 2
- WGQKYBSKWIADBV-UHFFFAOYSA-N benzylamine Chemical compound NCC1=CC=CC=C1 WGQKYBSKWIADBV-UHFFFAOYSA-N 0.000 description 2
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 2
- 150000001602 bicycloalkyls Chemical group 0.000 description 2
- 230000027455 binding Effects 0.000 description 2
- SIPUZPBQZHNSDW-UHFFFAOYSA-N bis(2-methylpropyl)aluminum Chemical compound CC(C)C[Al]CC(C)C SIPUZPBQZHNSDW-UHFFFAOYSA-N 0.000 description 2
- 239000006172 buffering agent Substances 0.000 description 2
- 125000004369 butenyl group Chemical group C(=CCC)* 0.000 description 2
- 210000004027 cell Anatomy 0.000 description 2
- IJOOHPMOJXWVHK-UHFFFAOYSA-N chlorotrimethylsilane Chemical compound C[Si](C)(C)Cl IJOOHPMOJXWVHK-UHFFFAOYSA-N 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- 239000012153 distilled water Substances 0.000 description 2
- 239000003995 emulsifying agent Substances 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 235000013922 glutamic acid Nutrition 0.000 description 2
- 239000004220 glutamic acid Substances 0.000 description 2
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 2
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 2
- 239000011630 iodine Substances 0.000 description 2
- 229910052740 iodine Inorganic materials 0.000 description 2
- 239000000865 liniment Substances 0.000 description 2
- 235000019421 lipase Nutrition 0.000 description 2
- 229910052744 lithium Inorganic materials 0.000 description 2
- KWGKDLIKAYFUFQ-UHFFFAOYSA-M lithium chloride Chemical compound [Li+].[Cl-] KWGKDLIKAYFUFQ-UHFFFAOYSA-M 0.000 description 2
- UBJFKNSINUCEAL-UHFFFAOYSA-N lithium;2-methylpropane Chemical compound [Li+].C[C-](C)C UBJFKNSINUCEAL-UHFFFAOYSA-N 0.000 description 2
- 208000019423 liver disease Diseases 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 229940095102 methyl benzoate Drugs 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 230000001151 other effect Effects 0.000 description 2
- 239000006187 pill Substances 0.000 description 2
- 229910000027 potassium carbonate Inorganic materials 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 125000004368 propenyl group Chemical group C(=CC)* 0.000 description 2
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 238000000159 protein binding assay Methods 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- NDVLTYZPCACLMA-UHFFFAOYSA-N silver oxide Chemical compound [O-2].[Ag+].[Ag+] NDVLTYZPCACLMA-UHFFFAOYSA-N 0.000 description 2
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 2
- 235000019345 sodium thiosulphate Nutrition 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 239000000829 suppository Substances 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- ILMRJRBKQSSXGY-UHFFFAOYSA-N tert-butyl(dimethyl)silicon Chemical group C[Si](C)C(C)(C)C ILMRJRBKQSSXGY-UHFFFAOYSA-N 0.000 description 2
- JLMWXJKOCRNXGG-RIYZIHGNSA-N tert-butyl-[(e)-4-iodo-1-(1-propylcyclobutyl)but-3-enoxy]-dimethylsilane Chemical compound I/C=C/CC(O[Si](C)(C)C(C)(C)C)C1(CCC)CCC1 JLMWXJKOCRNXGG-RIYZIHGNSA-N 0.000 description 2
- NHGXDBSUJJNIRV-UHFFFAOYSA-M tetrabutylammonium chloride Chemical compound [Cl-].CCCC[N+](CCCC)(CCCC)CCCC NHGXDBSUJJNIRV-UHFFFAOYSA-M 0.000 description 2
- 231100000419 toxicity Toxicity 0.000 description 2
- 230000001988 toxicity Effects 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- 210000003462 vein Anatomy 0.000 description 2
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 2
- 229920002554 vinyl polymer Polymers 0.000 description 2
- SDLIFPLGPYMCDT-VAVYLYDRSA-N (3s)-3-[tert-butyl(dimethyl)silyl]oxy-3-[(4r)-4-[tert-butyl(dimethyl)silyl]oxy-4-(1-propylcyclobutyl)but-1-enyl]-2-methylidenecyclopentan-1-one Chemical compound CCCC1([C@@H](CC=C[C@@]2(C(C(=O)CC2)=C)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)CCC1 SDLIFPLGPYMCDT-VAVYLYDRSA-N 0.000 description 1
- HNECKSLTVKQPGJ-CQSZACIVSA-N (4R)-4-[tert-butyl(dimethyl)silyl]oxy-2-(diethylamino)-3-methylcyclopent-2-en-1-one Chemical compound [Si](C)(C)(C(C)(C)C)O[C@H]1C(=C(C(C1)=O)N(CC)CC)C HNECKSLTVKQPGJ-CQSZACIVSA-N 0.000 description 1
- UEPOKNWIWRACBZ-AWEZNQCLSA-N (4r)-4-[tert-butyl(dimethyl)silyl]oxy-2-(diethylaminomethyl)cyclopent-2-en-1-one Chemical compound CCN(CC)CC1=C[C@H](O[Si](C)(C)C(C)(C)C)CC1=O UEPOKNWIWRACBZ-AWEZNQCLSA-N 0.000 description 1
- DAPZSGCXUJECAI-JTQLQIEISA-N (4r)-4-[tert-butyl(dimethyl)silyl]oxycyclopent-2-en-1-one Chemical compound CC(C)(C)[Si](C)(C)O[C@@H]1CC(=O)C=C1 DAPZSGCXUJECAI-JTQLQIEISA-N 0.000 description 1
- APQIUTYORBAGEZ-UHFFFAOYSA-N 1,1-dibromoethane Chemical compound CC(Br)Br APQIUTYORBAGEZ-UHFFFAOYSA-N 0.000 description 1
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 1
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 1
- LBLYYCQCTBFVLH-UHFFFAOYSA-N 2-Methylbenzenesulfonic acid Chemical compound CC1=CC=CC=C1S(O)(=O)=O LBLYYCQCTBFVLH-UHFFFAOYSA-N 0.000 description 1
- XWKFPIODWVPXLX-UHFFFAOYSA-N 2-methyl-5-methylpyridine Natural products CC1=CC=C(C)N=C1 XWKFPIODWVPXLX-UHFFFAOYSA-N 0.000 description 1
- WGTASENVNYJZBK-UHFFFAOYSA-N 3,4,5-trimethoxyamphetamine Chemical compound COC1=CC(CC(C)N)=CC(OC)=C1OC WGTASENVNYJZBK-UHFFFAOYSA-N 0.000 description 1
- DAPZSGCXUJECAI-UHFFFAOYSA-N 4-[tert-butyl(dimethyl)silyl]oxycyclopent-2-en-1-one Chemical compound CC(C)(C)[Si](C)(C)OC1CC(=O)C=C1 DAPZSGCXUJECAI-UHFFFAOYSA-N 0.000 description 1
- 229960000549 4-dimethylaminophenol Drugs 0.000 description 1
- OZAIFHULBGXAKX-VAWYXSNFSA-N AIBN Substances N#CC(C)(C)\N=N\C(C)(C)C#N OZAIFHULBGXAKX-VAWYXSNFSA-N 0.000 description 1
- 229920001450 Alpha-Cyclodextrin Polymers 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- HPWROEQUYBSPHB-HRBFZRADSA-N C#CCC(O/[3H]=B/S)C1(CCC)CCC1 Chemical compound C#CCC(O/[3H]=B/S)C1(CCC)CCC1 HPWROEQUYBSPHB-HRBFZRADSA-N 0.000 description 1
- XUEZDGLHSFJMKQ-CQIIZZFESA-N C.C=C1CC(O)[C@H](/C=C/CC(O)C2(C)CCC2)[C@H]1CCC1=CC=C(C(=O)O)C=C1 Chemical compound C.C=C1CC(O)[C@H](/C=C/CC(O)C2(C)CCC2)[C@H]1CCC1=CC=C(C(=O)O)C=C1 XUEZDGLHSFJMKQ-CQIIZZFESA-N 0.000 description 1
- QGECOMSHMSFQFS-YRGAYIMCSA-N C=C1CC(O)[C@H](/C=C/CC(O)C2(C)CCC2)C1SCC1=CC=C(C(=O)O)C=C1 Chemical compound C=C1CC(O)[C@H](/C=C/CC(O)C2(C)CCC2)C1SCC1=CC=C(C(=O)O)C=C1 QGECOMSHMSFQFS-YRGAYIMCSA-N 0.000 description 1
- XXTGEQVXFZZAQJ-XKHDZHNJSA-N C=C1CC(O)[C@H](/C=C/CC(O)C2(C)CCC2)[C@H]1CSC1=CC=C(C(=O)O)C=C1 Chemical compound C=C1CC(O)[C@H](/C=C/CC(O)C2(C)CCC2)[C@H]1CSC1=CC=C(C(=O)O)C=C1 XXTGEQVXFZZAQJ-XKHDZHNJSA-N 0.000 description 1
- IBBWEBAEAVAXOQ-WASBOHCDSA-N C=C1CC(OC)[C@H](/C=C/CC(O)C2(C)CCC2)[C@H]1CCC1=CC=C(C(=O)O)C=C1 Chemical compound C=C1CC(OC)[C@H](/C=C/CC(O)C2(C)CCC2)[C@H]1CCC1=CC=C(C(=O)O)C=C1 IBBWEBAEAVAXOQ-WASBOHCDSA-N 0.000 description 1
- DYXCQDIOOPXWSW-MHCSDRMFSA-N C=C1CC[C@H](/C=C/CC(O)C2(C)CCC2)[C@H]1CCC1=CC=C(C(=O)O)C=C1 Chemical compound C=C1CC[C@H](/C=C/CC(O)C2(C)CCC2)[C@H]1CCC1=CC=C(C(=O)O)C=C1 DYXCQDIOOPXWSW-MHCSDRMFSA-N 0.000 description 1
- IPMUZQIRWXEGSP-BIAZVWLNSA-M C=CCC1(C(O)C/C=C/[C@@H]2C(SCC3=CC=C(C(=O)OC)C=C3)=C(O[Ac])CC2O)CCC1 Chemical compound C=CCC1(C(O)C/C=C/[C@@H]2C(SCC3=CC=C(C(=O)OC)C=C3)=C(O[Ac])CC2O)CCC1 IPMUZQIRWXEGSP-BIAZVWLNSA-M 0.000 description 1
- UWDNWXVWXSESGM-NFRFTDDGSA-N C=CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound C=CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)O)C=C2)CCC1 UWDNWXVWXSESGM-NFRFTDDGSA-N 0.000 description 1
- PECWRRXQBVUYBK-PLVYPMBTSA-N C=CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)OC)C=C2)CCC1 Chemical compound C=CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)OC)C=C2)CCC1 PECWRRXQBVUYBK-PLVYPMBTSA-N 0.000 description 1
- QTNBKQGTOLZYMC-HULHONQRSA-N C=CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound C=CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 QTNBKQGTOLZYMC-HULHONQRSA-N 0.000 description 1
- UIWZEYLSMLJKJQ-CIWBHIKBSA-N C=CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)OC)C=C2)CCC1 Chemical compound C=CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)OC)C=C2)CCC1 UIWZEYLSMLJKJQ-CIWBHIKBSA-N 0.000 description 1
- WOGKGRRKMLISGL-MTJMWHMGSA-N C=CCC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound C=CCC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 WOGKGRRKMLISGL-MTJMWHMGSA-N 0.000 description 1
- DMPLEOOEPFYEIJ-KLOUOVKDSA-N C=CCC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)[C@@H]2CCC2=CC=C(C(=O)OC)C=C2)CCC1 Chemical compound C=CCC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)[C@@H]2CCC2=CC=C(C(=O)OC)C=C2)CCC1 DMPLEOOEPFYEIJ-KLOUOVKDSA-N 0.000 description 1
- GLEQLOLYILIJRI-ARIIAYFSSA-N CC(C)CC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CC(C)CC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)O)C=C2)CCC1 GLEQLOLYILIJRI-ARIIAYFSSA-N 0.000 description 1
- FWSVYCWFJJQYTB-FQYBEGEKSA-N CC(C)CC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CC(C)CC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 FWSVYCWFJJQYTB-FQYBEGEKSA-N 0.000 description 1
- SUCQMSOLMCCOTE-DEBVDFLFSA-N CC(C)CC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CC(C)CC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 SUCQMSOLMCCOTE-DEBVDFLFSA-N 0.000 description 1
- FHPRDHMMBCKAER-BCMMYDQPSA-N CC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)O)C=C2)CCC1 FHPRDHMMBCKAER-BCMMYDQPSA-N 0.000 description 1
- UADGPJUQTCRRED-BCBXONDCSA-N CC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 UADGPJUQTCRRED-BCBXONDCSA-N 0.000 description 1
- DOQGUQCJZDZPBL-ZHLVLLCMSA-N CC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CSC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CSC2=CC=C(C(=O)O)C=C2)CCC1 DOQGUQCJZDZPBL-ZHLVLLCMSA-N 0.000 description 1
- KXUADFXWMFITPG-OAHZBJFGSA-N CC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)C2SCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)C2SCC2=CC=C(C(=O)O)C=C2)CCC1 KXUADFXWMFITPG-OAHZBJFGSA-N 0.000 description 1
- GYHBXHCAZAJGMO-ZHZQXDIZSA-N CC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 GYHBXHCAZAJGMO-ZHZQXDIZSA-N 0.000 description 1
- FQBIEUGYTQAMQJ-OCWUVKRKSA-N CC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)[C@@H]2CSC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)[C@@H]2CSC2=CC=C(C(=O)O)C=C2)CCC1 FQBIEUGYTQAMQJ-OCWUVKRKSA-N 0.000 description 1
- STCCGWLSCNOJMM-XOAYCIQBSA-N CC1(C(O)C/C=C/[C@H]2CCC(=O)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CC1(C(O)C/C=C/[C@H]2CCC(=O)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 STCCGWLSCNOJMM-XOAYCIQBSA-N 0.000 description 1
- AIQQDTDOQBLFGN-NZMVLKDGSA-N CC1(C(O)C/C=C/[C@H]2CCC(Cl)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CC1(C(O)C/C=C/[C@H]2CCC(Cl)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 AIQQDTDOQBLFGN-NZMVLKDGSA-N 0.000 description 1
- XYFQMQUGBOAUEM-PCIIMRJMSA-M CCC1(C(O)C/C=C/[C@@H]2C(SCC3=CC=C(C(=O)OC)C=C3)=C(O[Ac])CC2O)CCC1 Chemical compound CCC1(C(O)C/C=C/[C@@H]2C(SCC3=CC=C(C(=O)OC)C=C3)=C(O[Ac])CC2O)CCC1 XYFQMQUGBOAUEM-PCIIMRJMSA-M 0.000 description 1
- YCESAVVRWKRMCS-PTZXROJESA-N CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)O)C=C2)CCC1 YCESAVVRWKRMCS-PTZXROJESA-N 0.000 description 1
- QVAJCCMAWZPJDP-RLXKOLJZSA-N CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)OC)C=C2)CCC1 Chemical compound CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)OC)C=C2)CCC1 QVAJCCMAWZPJDP-RLXKOLJZSA-N 0.000 description 1
- DSAFJVWSXZTKKA-GXNCQDSMSA-N CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 DSAFJVWSXZTKKA-GXNCQDSMSA-N 0.000 description 1
- RDILPVORRSPNOT-HRBKQULASA-N CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)OC)C=C2)CCC1 Chemical compound CCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)OC)C=C2)CCC1 RDILPVORRSPNOT-HRBKQULASA-N 0.000 description 1
- WLKCMRMYPDMGNT-YNGPYFOPSA-N CCC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CCC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 WLKCMRMYPDMGNT-YNGPYFOPSA-N 0.000 description 1
- CGXCEOWSHRGZIS-ACCUPZAFSA-N CCC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)[C@@H]2CCC2=CC=C(C(=O)OC)C=C2)CCC1 Chemical compound CCC1(C(O)C/C=C/[C@H]2C(O)CC(Cl)[C@@H]2CCC2=CC=C(C(=O)OC)C=C2)CCC1 CGXCEOWSHRGZIS-ACCUPZAFSA-N 0.000 description 1
- GSCFNZKEWDACGW-SLCZAINWSA-N CCCC1(C(C/C=C/I)O/[3H]=B/S)CCC1 Chemical compound CCCC1(C(C/C=C/I)O/[3H]=B/S)CCC1 GSCFNZKEWDACGW-SLCZAINWSA-N 0.000 description 1
- WHHOTWBMOROUCL-BIAZVWLNSA-M CCCC1(C(O)C/C=C/[C@@H]2C(SCC3=CC=C(C(=O)OC)C=C3)=C(O[Ac])CC2O)CCC1 Chemical compound CCCC1(C(O)C/C=C/[C@@H]2C(SCC3=CC=C(C(=O)OC)C=C3)=C(O[Ac])CC2O)CCC1 WHHOTWBMOROUCL-BIAZVWLNSA-M 0.000 description 1
- YGLGABGIGGVTMA-NFRFTDDGSA-N CCCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CCCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)O)C=C2)CCC1 YGLGABGIGGVTMA-NFRFTDDGSA-N 0.000 description 1
- YINYZFTXAAYJGQ-PLVYPMBTSA-N CCCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)OC)C=C2)CCC1 Chemical compound CCCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)C2SCC2=CC=C(C(=O)OC)C=C2)CCC1 YINYZFTXAAYJGQ-PLVYPMBTSA-N 0.000 description 1
- UKQBTMIUJAZTON-HULHONQRSA-N CCCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CCCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)O)C=C2)CCC1 UKQBTMIUJAZTON-HULHONQRSA-N 0.000 description 1
- GQXLJLRIWVQOAG-CIWBHIKBSA-N CCCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)OC)C=C2)CCC1 Chemical compound CCCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CCC2=CC=C(C(=O)OC)C=C2)CCC1 GQXLJLRIWVQOAG-CIWBHIKBSA-N 0.000 description 1
- HFZZGSWBMITMIM-GXNCQDSMSA-N CCCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CSC2=CC=C(C(=O)O)C=C2)CCC1 Chemical compound CCCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CSC2=CC=C(C(=O)O)C=C2)CCC1 HFZZGSWBMITMIM-GXNCQDSMSA-N 0.000 description 1
- WLDNHAWWHNLLLA-HRBKQULASA-N CCCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CSC2=CC=C(C(=O)OC)C=C2)CCC1 Chemical compound CCCC1(C(O)C/C=C/[C@H]2C(O)CC(=O)[C@@H]2CSC2=CC=C(C(=O)OC)C=C2)CCC1 WLDNHAWWHNLLLA-HRBKQULASA-N 0.000 description 1
- WHXKVABVMLPPPY-ORZGSPMASA-N COC(=O)C1=CC=C(CCC2=C(OC(C)=O)CC(O)[C@@H]2/C=C/CC(O)C2(C)CCC2)C=C1 Chemical compound COC(=O)C1=CC=C(CCC2=C(OC(C)=O)CC(O)[C@@H]2/C=C/CC(O)C2(C)CCC2)C=C1 WHXKVABVMLPPPY-ORZGSPMASA-N 0.000 description 1
- FNVHQHJOLVJAKZ-ZQVPANCKSA-N COC(=O)C1=CC=C(CCC2=C(OC(C)=O)CC(OC)[C@@H]2/C=C/CC(O)C2(C)CCC2)C=C1 Chemical compound COC(=O)C1=CC=C(CCC2=C(OC(C)=O)CC(OC)[C@@H]2/C=C/CC(O)C2(C)CCC2)C=C1 FNVHQHJOLVJAKZ-ZQVPANCKSA-N 0.000 description 1
- KBBJCZROUCJEPQ-XQVIEVRJSA-N COC(=O)C1=CC=C(CCC2=C(OC(C)=O)CC[C@@H]2/C=C/CC(O)C2(C)CCC2)C=C1 Chemical compound COC(=O)C1=CC=C(CCC2=C(OC(C)=O)CC[C@@H]2/C=C/CC(O)C2(C)CCC2)C=C1 KBBJCZROUCJEPQ-XQVIEVRJSA-N 0.000 description 1
- VJPAQJWKVLMEBU-ZORUNVSQSA-N COC(=O)C1=CC=C(CC[C@H]2C(=O)CC(O)[C@@H]2/C=C/CC(O)C2(CC(C)C)CCC2)C=C1 Chemical compound COC(=O)C1=CC=C(CC[C@H]2C(=O)CC(O)[C@@H]2/C=C/CC(O)C2(CC(C)C)CCC2)C=C1 VJPAQJWKVLMEBU-ZORUNVSQSA-N 0.000 description 1
- UFNPRJXIMSWERR-LORUBMJOSA-N COC(=O)C1=CC=C(CC[C@H]2C(=O)CC(O)[C@@H]2/C=C/CC(O)C2(CC3CC3)CCC2)C=C1 Chemical compound COC(=O)C1=CC=C(CC[C@H]2C(=O)CC(O)[C@@H]2/C=C/CC(O)C2(CC3CC3)CCC2)C=C1 UFNPRJXIMSWERR-LORUBMJOSA-N 0.000 description 1
- SXPLAZIMRGSXEP-ZKNZBWHASA-N COC(=O)C1=CC=C(CC[C@H]2C(Cl)CC(O)[C@@H]2/C=C/CC(O)C2(CC(C)C)CCC2)C=C1 Chemical compound COC(=O)C1=CC=C(CC[C@H]2C(Cl)CC(O)[C@@H]2/C=C/CC(O)C2(CC(C)C)CCC2)C=C1 SXPLAZIMRGSXEP-ZKNZBWHASA-N 0.000 description 1
- KGVLBYCFXSKYCY-GHANZFMESA-N COC(=O)C1=CC=C(CC[C@H]2C(O[Ac])CC(O)[C@@H]2/C=C/CC(O)C2(CC3CC3)CCC2)C=C1 Chemical compound COC(=O)C1=CC=C(CC[C@H]2C(O[Ac])CC(O)[C@@H]2/C=C/CC(O)C2(CC3CC3)CCC2)C=C1 KGVLBYCFXSKYCY-GHANZFMESA-N 0.000 description 1
- FHYCJIJRLMJEMF-AGIHJTPKSA-N COC(=O)C1=CC=C(CSC2=C(OC(C)=O)CC(O)[C@@H]2/C=C/CC(O)C2(C)CCC2)C=C1 Chemical compound COC(=O)C1=CC=C(CSC2=C(OC(C)=O)CC(O)[C@@H]2/C=C/CC(O)C2(C)CCC2)C=C1 FHYCJIJRLMJEMF-AGIHJTPKSA-N 0.000 description 1
- KORBRTWRNFIHLA-MTJXOJBFSA-M COC(=O)C1=CC=C(CSC2=C(O[Ac])CC(O)[C@@H]2/C=C/CC(O)C2(CC(C)C)CCC2)C=C1 Chemical compound COC(=O)C1=CC=C(CSC2=C(O[Ac])CC(O)[C@@H]2/C=C/CC(O)C2(CC(C)C)CCC2)C=C1 KORBRTWRNFIHLA-MTJXOJBFSA-M 0.000 description 1
- SKSQFLRMRUGRJH-INDKINSYSA-M COC(=O)C1=CC=C(CSC2=C(O[Ac])CC(O)[C@@H]2/C=C/CC(O)C2(CC3CC3)CCC2)C=C1 Chemical compound COC(=O)C1=CC=C(CSC2=C(O[Ac])CC(O)[C@@H]2/C=C/CC(O)C2(CC3CC3)CCC2)C=C1 SKSQFLRMRUGRJH-INDKINSYSA-M 0.000 description 1
- KQVJTDDAEOKEHK-GWRRSUKYSA-N COC(=O)C1=CC=C(CSC2C(=O)CC(O)[C@@H]2/C=C/CC(O)C2(CC(C)C)CCC2)C=C1 Chemical compound COC(=O)C1=CC=C(CSC2C(=O)CC(O)[C@@H]2/C=C/CC(O)C2(CC(C)C)CCC2)C=C1 KQVJTDDAEOKEHK-GWRRSUKYSA-N 0.000 description 1
- GGOZGILGHCBTHM-CDKNWKFZSA-N COC(=O)C1=CC=C(CSC2C(=O)CC(O)[C@@H]2/C=C/CC(O)C2(CC3CC3)CCC2)C=C1 Chemical compound COC(=O)C1=CC=C(CSC2C(=O)CC(O)[C@@H]2/C=C/CC(O)C2(CC3CC3)CCC2)C=C1 GGOZGILGHCBTHM-CDKNWKFZSA-N 0.000 description 1
- CGPUWFMAIPBRIJ-OLGIVPTFSA-N COC(=O)C1=CC=C(SCC2=C(OC(C)=O)CC(O)[C@@H]2/C=C/CC(O)C2(C)CCC2)C=C1 Chemical compound COC(=O)C1=CC=C(SCC2=C(OC(C)=O)CC(O)[C@@H]2/C=C/CC(O)C2(C)CCC2)C=C1 CGPUWFMAIPBRIJ-OLGIVPTFSA-N 0.000 description 1
- VKSOYWMQGOOIDJ-GEGZGGFKSA-N COC1CC(=O)[C@H](CCC2=CC=C(C(=O)O)C=C2)[C@H]1/C=C/CC(O)C1(C)CCC1 Chemical compound COC1CC(=O)[C@H](CCC2=CC=C(C(=O)O)C=C2)[C@H]1/C=C/CC(O)C1(C)CCC1 VKSOYWMQGOOIDJ-GEGZGGFKSA-N 0.000 description 1
- NJMNWOURRHMTFN-YNGPYFOPSA-N COC1CC(Cl)[C@H](CCC2=CC=C(C(=O)O)C=C2)[C@H]1/C=C/CC(O)C1(C)CCC1 Chemical compound COC1CC(Cl)[C@H](CCC2=CC=C(C(=O)O)C=C2)[C@H]1/C=C/CC(O)C1(C)CCC1 NJMNWOURRHMTFN-YNGPYFOPSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 238000006845 Michael addition reaction Methods 0.000 description 1
- 101100029148 Mus musculus Ptger1 gene Proteins 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- NOUGVEGSWHWCMZ-AFRQWXMGSA-N O=C(O)C1=CC=C(CC[C@H]2C(=O)CC(O)[C@@H]2/C=C/CC(O)C2(CC3CC3)CCC2)C=C1 Chemical compound O=C(O)C1=CC=C(CC[C@H]2C(=O)CC(O)[C@@H]2/C=C/CC(O)C2(CC3CC3)CCC2)C=C1 NOUGVEGSWHWCMZ-AFRQWXMGSA-N 0.000 description 1
- UXVZYCPEYRIMFD-FTGCYEOKSA-N O=C(O)C1=CC=C(CSC2C(=O)CC(O)[C@@H]2/C=C/CC(O)C2(CC3CC3)CCC2)C=C1 Chemical compound O=C(O)C1=CC=C(CSC2C(=O)CC(O)[C@@H]2/C=C/CC(O)C2(CC3CC3)CCC2)C=C1 UXVZYCPEYRIMFD-FTGCYEOKSA-N 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 241000238370 Sepia Species 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 235000010724 Wisteria floribunda Nutrition 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- RRIURAFFBKBUPG-FSEIIBCTSA-N [3H]=BSOC1C=C(SCC2=CC=C(C(=O)OC)C=C2)C(=O)C1 Chemical compound [3H]=BSOC1C=C(SCC2=CC=C(C(=O)OC)C=C2)C(=O)C1 RRIURAFFBKBUPG-FSEIIBCTSA-N 0.000 description 1
- QPTIIQQBKBFAAK-UAILKZIDSA-N [3H]=BSOC1CC(=O)C(=C)[C@H]1/C=C/CC(O/[3H]=B/S)C1(CCC)CCC1 Chemical compound [3H]=BSOC1CC(=O)C(=C)[C@H]1/C=C/CC(O/[3H]=B/S)C1(CCC)CCC1 QPTIIQQBKBFAAK-UAILKZIDSA-N 0.000 description 1
- LZOIAWZLGJEWCC-CKAQZFCNSA-N [3H]=BSOC1CC(=O)[C@H](CCC2=CC=C(C(=O)OC)C=C2)[C@H]1/C=C/CC(O/[3H]=B/S)C1(CCC)CCC1 Chemical compound [3H]=BSOC1CC(=O)[C@H](CCC2=CC=C(C(=O)OC)C=C2)[C@H]1/C=C/CC(O/[3H]=B/S)C1(CCC)CCC1 LZOIAWZLGJEWCC-CKAQZFCNSA-N 0.000 description 1
- ZZYVVHOCOANAOR-DXIPQQAESA-N [3H]=BSOC1CC(=O)[C@H](CSC2=CC=C(C(=O)OC)C=C2)[C@H]1/C=C/CC(O/[3H]=B/S)C1(CCC)CCC1 Chemical compound [3H]=BSOC1CC(=O)[C@H](CSC2=CC=C(C(=O)OC)C=C2)[C@H]1/C=C/CC(O/[3H]=B/S)C1(CCC)CCC1 ZZYVVHOCOANAOR-DXIPQQAESA-N 0.000 description 1
- CFUSRTDHYNCCHY-ASZAMMKQSA-N [3H]=BSOC1CC(Cl)[C@H](CCC2=CC=C(C(=O)OC)C=C2)[C@H]1/C=C/CC(O/[3H]=B/S)C1(CC2CC2)CCC1 Chemical compound [3H]=BSOC1CC(Cl)[C@H](CCC2=CC=C(C(=O)OC)C=C2)[C@H]1/C=C/CC(O/[3H]=B/S)C1(CC2CC2)CCC1 CFUSRTDHYNCCHY-ASZAMMKQSA-N 0.000 description 1
- DAKPMYGRKIMAHG-UMOOHAHVSA-M [3H]=BSOC1CC(O[Ac])=C(SCC2=CC=C(C(=O)OC)C=C2)[C@H]1/C=C/CC(O/[3H]=B/S)C1(CC)CCC1 Chemical compound [3H]=BSOC1CC(O[Ac])=C(SCC2=CC=C(C(=O)OC)C=C2)[C@H]1/C=C/CC(O/[3H]=B/S)C1(CC)CCC1 DAKPMYGRKIMAHG-UMOOHAHVSA-M 0.000 description 1
- JNDGSQLSHDOJKH-JWEGSUJWSA-N [3H]=BSOC1C[C@H](C)[C@H](CCC2=CC=C(C(=O)OC)C=C2)[C@H]1/C=C/CC(O/[3H]=B/S)C1(CC2CC2)CCC1 Chemical compound [3H]=BSOC1C[C@H](C)[C@H](CCC2=CC=C(C(=O)OC)C=C2)[C@H]1/C=C/CC(O/[3H]=B/S)C1(CC2CC2)CCC1 JNDGSQLSHDOJKH-JWEGSUJWSA-N 0.000 description 1
- WLLIXJBWWFGEHT-UHFFFAOYSA-N [tert-butyl(dimethyl)silyl] trifluoromethanesulfonate Chemical compound CC(C)(C)[Si](C)(C)OS(=O)(=O)C(F)(F)F WLLIXJBWWFGEHT-UHFFFAOYSA-N 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 150000001351 alkyl iodides Chemical class 0.000 description 1
- 125000000304 alkynyl group Chemical group 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- HFHDHCJBZVLPGP-RWMJIURBSA-N alpha-cyclodextrin Chemical compound OC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)CO)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1CO HFHDHCJBZVLPGP-RWMJIURBSA-N 0.000 description 1
- 229940043377 alpha-cyclodextrin Drugs 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- AGEZXYOZHKGVCM-UHFFFAOYSA-N benzyl bromide Chemical compound BrCC1=CC=CC=C1 AGEZXYOZHKGVCM-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- WHGYBXFWUBPSRW-FOUAGVGXSA-N beta-cyclodextrin Chemical compound OC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)CO)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1CO WHGYBXFWUBPSRW-FOUAGVGXSA-N 0.000 description 1
- 229960004853 betadex Drugs 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 125000000480 butynyl group Chemical group [*]C#CC([H])([H])C([H])([H])[H] 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- CHRHZFQUDFAQEQ-UHFFFAOYSA-L calcium;2-hydroxyacetate Chemical compound [Ca+2].OCC([O-])=O.OCC([O-])=O CHRHZFQUDFAQEQ-UHFFFAOYSA-L 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 229940084030 carboxymethylcellulose calcium Drugs 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 238000013375 chromatographic separation Methods 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000007891 compressed tablet Substances 0.000 description 1
- 230000037020 contractile activity Effects 0.000 description 1
- DOBRDRYODQBAMW-UHFFFAOYSA-N copper(i) cyanide Chemical compound [Cu+].N#[C-] DOBRDRYODQBAMW-UHFFFAOYSA-N 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000000582 cycloheptyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- NISGSNTVMOOSJQ-UHFFFAOYSA-N cyclopentanamine Chemical compound NC1CCCC1 NISGSNTVMOOSJQ-UHFFFAOYSA-N 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 230000001120 cytoprotective effect Effects 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 230000001079 digestive effect Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 230000001882 diuretic effect Effects 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 125000005448 ethoxyethyl group Chemical group [H]C([H])([H])C([H])([H])OC([H])([H])C([H])([H])* 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- GDSRMADSINPKSL-HSEONFRVSA-N gamma-cyclodextrin Chemical compound OC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)CO)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1CO GDSRMADSINPKSL-HSEONFRVSA-N 0.000 description 1
- 229940080345 gamma-cyclodextrin Drugs 0.000 description 1
- 230000027119 gastric acid secretion Effects 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 235000001727 glucose Nutrition 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 239000007902 hard capsule Substances 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000006038 hexenyl group Chemical group 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000005980 hexynyl group Chemical group 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 150000002431 hydrogen Chemical class 0.000 description 1
- 229920003132 hydroxypropyl methylcellulose phthalate Polymers 0.000 description 1
- 229940031704 hydroxypropyl methylcellulose phthalate Drugs 0.000 description 1
- 230000001077 hypotensive effect Effects 0.000 description 1
- 239000005457 ice water Substances 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 239000010410 layer Substances 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- SNHOZPMHMQQMNI-UHFFFAOYSA-N lithium;2h-thiophen-2-ide Chemical compound [Li+].C=1C=[C-]SC=1 SNHOZPMHMQQMNI-UHFFFAOYSA-N 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- HCWCAKKEBCNQJP-UHFFFAOYSA-N magnesium orthosilicate Chemical compound [Mg+2].[Mg+2].[O-][Si]([O-])([O-])[O-] HCWCAKKEBCNQJP-UHFFFAOYSA-N 0.000 description 1
- 239000000391 magnesium silicate Substances 0.000 description 1
- 229910052919 magnesium silicate Inorganic materials 0.000 description 1
- 235000019792 magnesium silicate Nutrition 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- BGOSMQQLYYSHAU-MRXNPFEDSA-N methyl 4-[[(3r)-3-[tert-butyl(dimethyl)silyl]oxy-5-oxocyclopenten-1-yl]sulfanylmethyl]benzoate Chemical compound C1=CC(C(=O)OC)=CC=C1CSC1=C[C@H](O[Si](C)(C)C(C)(C)C)CC1=O BGOSMQQLYYSHAU-MRXNPFEDSA-N 0.000 description 1
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 239000012046 mixed solvent Substances 0.000 description 1
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- 230000009871 nonspecific binding Effects 0.000 description 1
- 125000004365 octenyl group Chemical group C(=CCCCCCC)* 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000005069 octynyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C#C* 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 125000002255 pentenyl group Chemical group C(=CCCC)* 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 125000005981 pentynyl group Chemical group 0.000 description 1
- 239000002304 perfume Substances 0.000 description 1
- 230000008855 peristalsis Effects 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910000160 potassium phosphate Inorganic materials 0.000 description 1
- 235000011009 potassium phosphates Nutrition 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 125000002568 propynyl group Chemical group [*]C#CC([H])([H])[H] 0.000 description 1
- 230000002997 prostaglandinlike Effects 0.000 description 1
- 239000008213 purified water Substances 0.000 description 1
- GRJJQCWNZGRKAU-UHFFFAOYSA-N pyridin-1-ium;fluoride Chemical compound F.C1=CC=NC=C1 GRJJQCWNZGRKAU-UHFFFAOYSA-N 0.000 description 1
- ZDYVRSLAEXCVBX-UHFFFAOYSA-N pyridinium p-toluenesulfonate Chemical compound C1=CC=[NH+]C=C1.CC1=CC=C(S([O-])(=O)=O)C=C1 ZDYVRSLAEXCVBX-UHFFFAOYSA-N 0.000 description 1
- 238000001953 recrystallisation Methods 0.000 description 1
- 239000012266 salt solution Substances 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 229910001923 silver oxide Inorganic materials 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- 239000007901 soft capsule Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000012289 standard assay Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- VRNAKYCHGZYXOA-UHFFFAOYSA-N tert-butyl-[1-(1-ethylcyclobutyl)-4-iodobut-3-enoxy]-dimethylsilane Chemical compound IC=CCC(O[Si](C)(C)C(C)(C)C)C1(CC)CCC1 VRNAKYCHGZYXOA-UHFFFAOYSA-N 0.000 description 1
- UNRXEMQBSHJQBB-UHFFFAOYSA-N tert-butyl-dimethyl-[1-(1-propylcyclobutyl)but-3-ynoxy]silane Chemical compound CC(C)(C)[Si](C)(C)OC(CC#C)C1(CCC)CCC1 UNRXEMQBSHJQBB-UHFFFAOYSA-N 0.000 description 1
- BCNZYOJHNLTNEZ-UHFFFAOYSA-N tert-butyldimethylsilyl chloride Chemical compound CC(C)(C)[Si](C)(C)Cl BCNZYOJHNLTNEZ-UHFFFAOYSA-N 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- 125000004187 tetrahydropyran-2-yl group Chemical group [H]C1([H])OC([H])(*)C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- QEMXHQIAXOOASZ-UHFFFAOYSA-N tetramethylammonium Chemical compound C[N+](C)(C)C QEMXHQIAXOOASZ-UHFFFAOYSA-N 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 229930192474 thiophene Natural products 0.000 description 1
- DBGVGMSCBYYSLD-UHFFFAOYSA-N tributylstannane Chemical compound CCCC[SnH](CCCC)CCCC DBGVGMSCBYYSLD-UHFFFAOYSA-N 0.000 description 1
- YNJBWRMUSHSURL-UHFFFAOYSA-N trichloroacetic acid Chemical compound OC(=O)C(Cl)(Cl)Cl YNJBWRMUSHSURL-UHFFFAOYSA-N 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 125000002221 trityl group Chemical group [H]C1=C([H])C([H])=C([H])C([H])=C1C([*])(C1=C(C(=C(C(=C1[H])[H])[H])[H])[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 238000010792 warming Methods 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C405/00—Compounds containing a five-membered ring having two side-chains in ortho position to each other, and having oxygen atoms directly attached to the ring in ortho position to one of the side-chains, one side-chain containing, not directly attached to the ring, a carbon atom having three bonds to hetero atoms with at the most one bond to halogen, and the other side-chain having oxygen atoms attached in gamma-position to the ring, e.g. prostaglandins ; Analogues or derivatives thereof
Definitions
- This invention relates to ⁇ -cycloalkyl-prostaglandin E 2 derivatives, a process for the preparation thereof, and a pharmaceutical agent containing it as an active ingredient. More particularly, this invention relates to
- Prostaglandin E 2 (abbreviated as PGE 2 hereinafter) has been known as a metabolite in the arachidonic acid cascade. It has been known that PGE 2 has cyto-protective activity, uterine contractile activity, a pain-inducing effect, a promoting effect of digestive peristalsis, an awakening effect, a suppressive effect of gastric acid secretion, hypotensive activity and diuretic activity etc.
- PGE 2 receptor was divided into some subtypes which possess different physiological roles each other. At present, four receptor subtypes are known and they are called EP1, EP2, EP3 and EP4 (Negishi M. et al., J. Lipid Mediators Cell Signaling, 12, 379-391 (1995)).
- the present inventors investigated to find new compounds which bind on each receptor specifically, so that we found that the compounds of the present invention could bind strongly on EP2 subtype receptor and achieved the present invention.
- immunological diseases autoimmune diseases, organ transplantation etc.
- asthma abnormal bone formation
- neuronal cell death hepatopathy
- abortion premature birth or retina neuropathy of glaucoma etc.
- R 1A and R 2A are hydrogen; R 3A is hydrogen or is taken together with R 4A to form a methylene chain of 4 carbon atoms wherein a cycloalkyl of 6 carbon atoms inclusive is formed, or is taken together with R 4A to form a bicycloalkenyl or bicycloalkyl moiety having the formula
- R 4A together with R 3A forms a cycloalkyl, bicycloalkyl or bicycloalkenyl as defined above, or together with R 5A forms a methylene chain of 3 carbon atoms wherein a cycloalkyl of 4 carbon atoms inclusive is formed;
- R 5A is hydrogen, or is taken together with R to form a cycloalkyl as defined above; and
- R 6A is hydrogen or straight-chain alkyl having 8 carbon atoms.
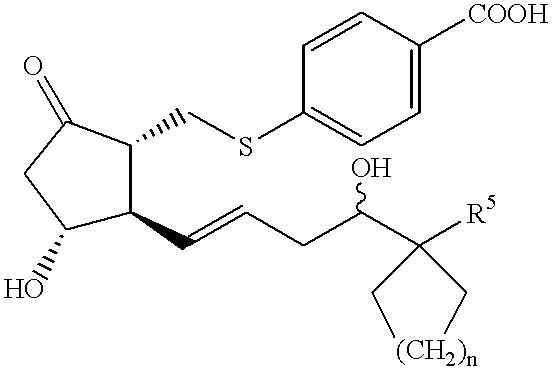
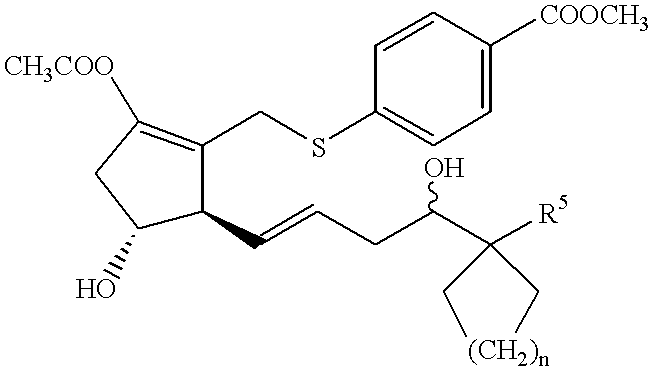
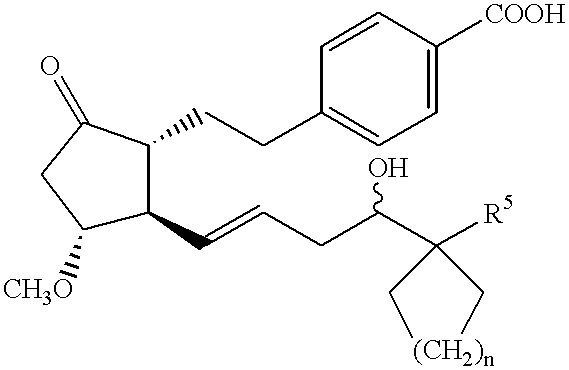
- the present invention relates to
- R 1 is hydroxy, C1-6 alkoxy or a group of formula
- R 10 and R 11 are each independently, hydrogen atom or C1-4 alkyl
- R 2 is C1-4 alkylene, C2-4 alkenylene, —S—C1-4 alkylene, —S—C2-4 alkenylene or C1-4 alkylene-S—;
- R 3 is oxo, methylene, halogen atom or a group of formula
- R 32 is C1-4 alkyl, C1-4 alkoxy, phenyl, phenyl-C1-4 alkyl,
- R 33 OOC—C1-4 alkyl or R 33 —OOC—C2-4 alkenyl (wherein R 33 is hydrogen atom or C1-4 alkyl);
- R 4 is hydrogen atom, hydroxy or C1-4 alkoxy
- R 5 is C1-8 alkyl, C2-8 alkenyl, C2-8 alkynyl, or C1-8 alkyl, C2-8 alkenyl or C2-8 alkynyl substituted by 1-3 substituents selected from (1)-(5) below:
- n 0-4;
- R 3 is R 32 —COO—, and R 1 is C1-6 alkoxy
- C1-4 alkyl in the definitions of R 11 , R 12 , R 32 , R 33 and R 5 means methyl, ethyl, propyl, butyl and isomers thereof.
- C1-8 alkyl in the definitions of Rs means methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl and isomers thereof.
- C1-4 alkoxy represented by R 32 , R 4 and Rs means methoxy, ethoxy, propoxy, butoxy and isomers thereof.
- C1-6 alkoxy representedby R′ means methoxy, ethoxy, propoxy, butoxy, pentyloxy, hexyloxy and isomers thereof.
- C2-4 alkenylin the definitions of R 32 means vinyl, propenyl, butenyl and isomers thereof.
- C1-4 alkylene represented by R 2 is methylene, dimethylene, trimethylene, tetramethylene and isomers thereof.
- R 2 is vinylene, propenylene, butenylene and isomers thereof.
- C2-8 alkenyl represented by R 3 means vinyl, propenyl, butenyl, pentenyl, hexenyl, heptenyl, octenyl and isomers thereof.
- C2-8 alkynyl representedby R5 means ethynyl, propynyl, butynyl, pentynyl, hexynyl, heptynyl, octynyl and isomers thereof.
- C3-7 cycloalkyl in the definitions of R 5 means cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl and cycloheptyl.
- halogen atom in the definitions of R 3 and R 5 means fluorine, chlorine, bromine and iodine.
- the substituent attached thereto may be in front of or behind the sheet or may be a mixture of isomers in front of and behind the sheet.
- alkyl, alkenyl and alkynyl groups include straight-chain and also branched-chain ones.
- the double bond in alkenyl group includes E, Z and EZ mixed isomers. Isomers resulting from the presence of asymmetric carbon atom(s) e.g. in branched-chain alkyl are included in the present invention.
- Preferred compounds of the present invention include compounds of the formula (I) listed in the examples or in Tables 1-20 below.
- the compounds of the present invention of formula (I) may be converted into the corresponding salts by a conventional means.
- Non-toxic, and water-soluble salts are preferable.
- Appropriate salts are described below; salts of alkali metals (potassium, sodium etc.), salts of alkaline-earth metals (calcium, magnesium etc.), ammonium salts, salts of pharmaceutically acceptable organic amines (tetramethyl ammonium, triethylamine, methylamine, dimethylamine, cyclopentylamine, benzylamine, phenethylamine, piperidine, monoethanolamine, diethanolamine, tris(hydroxymethyl)methylamine, lysine, arginine, N-methyl-D-glucamine etc.).
- Prostanic acid derivatives of formula (I) may be converted into cyclodextrin clathrates using ⁇ -, ⁇ - or ⁇ -cyclodextrin or a mixture thereof, by the methods described in the specification of Japanese Kokoku No.50-3363 or Japanese Kokoku No.52-31404 (i.e. GB Patent Nos. 1351238 or 1419221). Converting into their cyclodextrin clathrates serves to increase the stability and solubility in water of the compounds, and therefore it is useful in the use for pharmaceuticals.
- R 30 is oxo, methylene or halogen atom, and the other symbols are as defined above
- R 12 is C1-6 alkyl, and the other symbols are as defined above).
- Hydrolysis using an enzyme is known, for example, it may be carried out in a mixture of a water-miscible organic solvent (ethanol, dimethylsulfoxide etc.) and water, in the presence or absence of buffer, using an ester-cleaving enzyme (esterase, lipase etc.) at a temperature of from 0° C. to 50° C.
- a water-miscible organic solvent ethanol, dimethylsulfoxide etc.
- buffer ethanol, dimethylsulfoxide etc.
- ester-cleaving enzyme esterase, lipase etc.
- Hydrolysis under alkaline conditions is known, for example, it may be carried out in awater-miscible organic solvent (ethanol, tetrahydrofuran (THF), dioxane etc.) using an aqueous solution of an alkali (sodium hydroxide, potassium hydroxide, potassium carbonate etc.) at a temperature of from ⁇ 10° C. to 90° C.
- awater-miscible organic solvent ethanol, tetrahydrofuran (THF), dioxane etc.
- an alkali sodium hydroxide, potassium hydroxide, potassium carbonate etc.
- Amidation is known, for example, it may be carried out in an inert organic solvent (THF, methylene chloride, benzene, acetone, acetonitrile or a mixture thereof etc.), in the presence or absence of a tertiary amine (dimethylaminopyridine, pyridine, triethylamine etc.), using a condensing agent (1,3-dicyclohexylcarbodiimide (DCC), 1-ethyl-3-[3-(dimethylamino) propyl]carbodiimide (EDC) etc.) at a temperature of from 0° C. to 50° C.
- THF inert organic solvent
- benzene acetone
- DCC dimethylaminopyridine
- EDC 1-ethyl-3-[3-(dimethylamino) propyl]carbodiimide
- R 40 is hydrogen atom or hydroxy, and the other symbols are as defined above
- R 41 is hydrogen atom or hydroxy protected by a protective group which may be removed under acidic conditions
- R 60 is a protective group of hydroxy which may be removed under acidic conditions, and the other symbols are as defined above.
- a protective group of hydroxy which may be removed under acidic conditions includes, for example, t-butyldimethylsilyl, triphenylmethyl, tetrahydropyran-2-yl etc.
- Hydrolysis under acidic conditions is known; for example, it may be carried out in a water-miscible organic solvent (tetrahydrofuran, methanol, ethanol, dimethoxyethane, acetonitrile or a mixture thereof etc.) using an inorganic acid (hydrochloric acid, phosphoric acid, hydrofluoric acid, hydrofluoric acid-pyridine complex etc.) or an organic acid (acetic acid, toluenesulfonic acid, trichloroacetic acid etc.), at a temperature of from 0° C. to 50° C.
- a water-miscible organic solvent tetrahydrofuran, methanol, ethanol, dimethoxyethane, acetonitrile or a mixture thereof etc.
- an inorganic acid hydroochloric acid, phosphoric acid, hydrofluoric acid, hydrofluoric acid-pyridine complex etc.
- organic acid acetic acid, toluenesulfonic acid,
- R 42 is C1-4 alkoxy and the other symbols are as defined above
- R 42 may be prepared by O-alkylation of a compound of formula (IB) wherein R 4 is hydroxy, i.e. a compound of formula (IB-3)
- O-Alkylation is known, for example, it may be carried out in an inert organic solvent (THF, diethyl ether etc.), using diazoalkane at a temperature of from ⁇ 30° C. to 40° C. or in an inert organic solvent (acetonitrile etc.), in the presence of silver oxide, using alkyl iodide at a temperature of from 0° C. to 40° C.
- THF inert organic solvent
- diethyl ether etc. diethyl ether etc.
- diazoalkane at a temperature of from ⁇ 30° C. to 40° C.
- an inert organic solvent acetonitrile etc.
- Deprotection reaction may be carried out as described above.
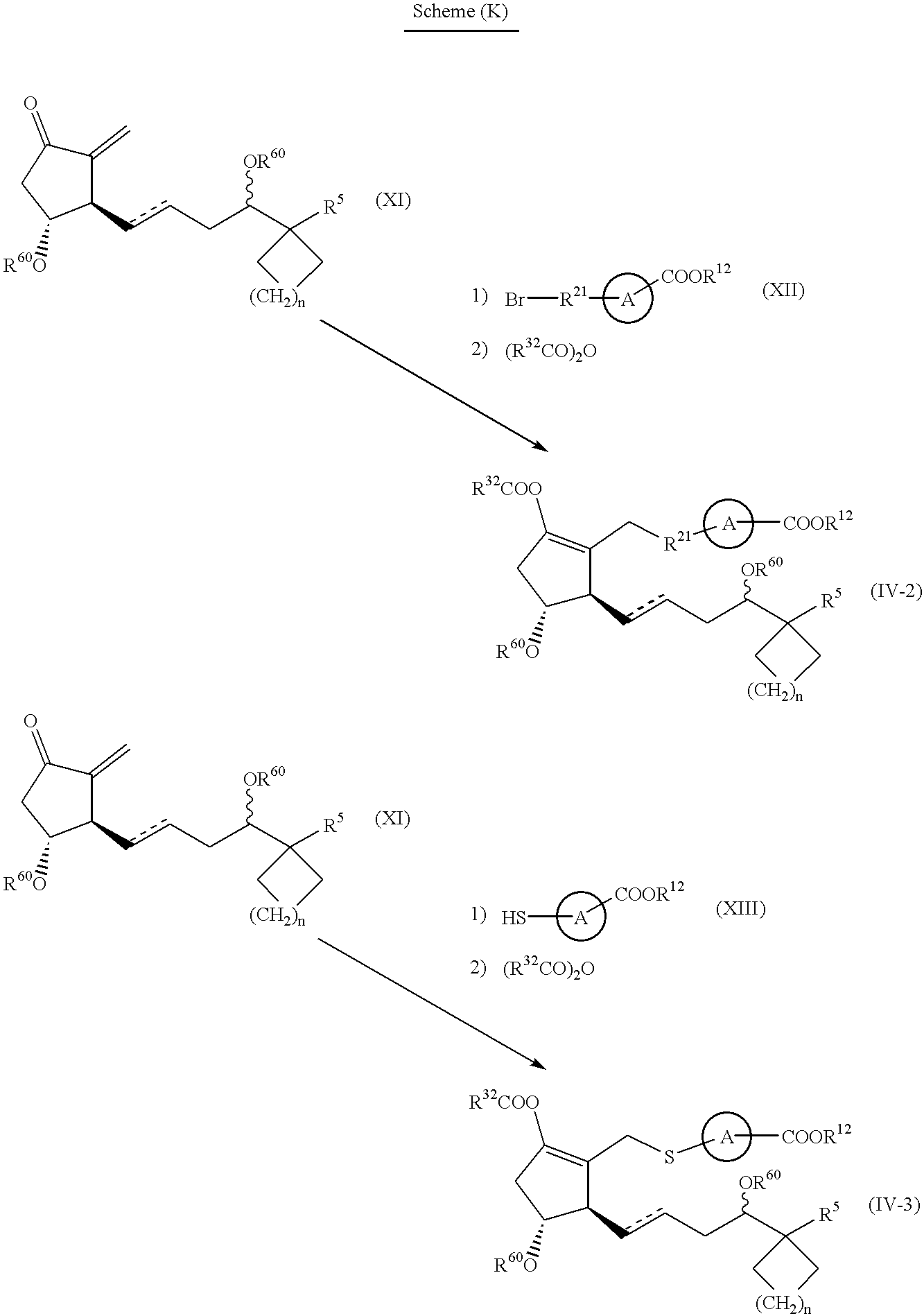
- a compound of formula (IV) may be prepared by scheme (G) or (K) described below.
- a compound of formula (III) may be divided into the following six classes of compounds according to the values of R 30 and R 41 . That is,
- R 30 is oxo and R 41 is hydroxy protected by a protective group which may be removed under acidic conditions, i.e. a compound of formula (IIIA)
- R 30 is methylene and R 41 is hydroxy protected by a protective group which may be removed under acidic conditions, i.e. a compound of formula (IIIB)
- R 30 is halogen atom and R 41 is hydroxy protected by a protective group which may be removable under acidic conditions, i.e. a compound of formula (IIIC)
- R 31 is halogen atom, and the other symbols are as defined above).
- R 30 is oxo;
- R 41 is hydrogen atom, i.e. a compound of formula (IIID)
- R 30 is methylene and R 41 is hydrogen atom, i.e. a compound of formula (IIIE)
- R 30 is halogen atom and R 41 is hydrogen atom, i.e. a compound of formula (IIIF)
- a compound of formula (IIIB) may be prepared from a compound of formula (IIIA) according to the reaction of the following Scheme (A).
- a compound of formula (IIIC) may be prepared from a compound of formula (IIIA) according to the reactions of the following Scheme (B), (C) or (D).
- a compound of formula (IIID) may be prepared from a compound of formula (IIA) according to the reactions of the following Scheme (E).
- a compound of formula (IIIE) may be prepared from a compound of formula (IIID) according to the same reactions as the following Scheme (A).
- a compound of formula (IIIF) may be prepared from a compound of formula (IIID) according to the same reactions as the following Scheme (B), (C) or (D).
- a compound of formula (IIIA) may be prepared according to the reactions of the following Scheme (F), (G), (H) or (J).
- R 21 is C1-3 alkylene or C2-3 alkenylene
- R 22 is C1-3 alkylene
- t-Bu is t-butyl
- n-Bu is normal butyl
- c-Hex is cyclohexyl
- Et is ethyl
- EE is ethoxyethyl
- Ph is phenyl
- Ts is p-toluenesulfonyl
- Ms is methanesulfonyl
- DMAP is dimethylaminopyridine
- AIBN is 2,2′-azobisisobutyronitrile
- DIBAL is diisobutylaluminum hydride
- reaction in reaction Schemes described above is carried out by known methods.
- the compounds of formula (V), (VII), (VIII), (IX), (X), (XII), (XIII) and (XIV) as starting materials are known per se or may be prepared by known methods.
- (4RS) -5,5-propanooct-1-yn-4-ol is a known compound described in the specification of JP54-115351.
- the other starting materials and reagents in the present invention are known per se or may be prepared by known methods.
- obtained products may be purified by conventional techniques.
- purification maybe carried out by distillation under atmospheric or reduced pressure, by high performance liquid chromatography, by thin layer chromatography or by column chromatography using silica gel or magnesium silicate, by washing or by recrystallization. Purification may be carried out after each reaction, or after a series of reactions.
- the compounds of the present invention of formula (I) bind and act on EP2 receptor which is a subtype of PGE 2 receptor.
- the effects of the compounds of the present invention were confirmed by binding assay using expression cell of prostanoids receptor subtype.
- membrane fraction was carried out according to the method of Sugimoto et. al., [J. Biol. Chem., 267, 6463-6466 (1992)], using expression CHO cell of the prostanoids receptor subtype (mouse EP1, EP2, EP3 ⁇ , EP4).
- Buffer 10 mM potassium phosphate (pH 6.0), 1 mM EDTA, 10 mM MgCl 2 , 0.1 M NaCl.
- Ki IC 50 /(1+[C]/Kd))
- the toxicity of the compounds of the present invention is very low and therefore, it is confirmed that these compounds are safe for pharmaceutical use.
- the compounds of the present invention of formula (I) bind strongly and act on PGE 2 receptor, especially on EP2 subtype receptor and therefore are useful for prevention and/or treatment of immunological diseases (autoimmune diseases, organ transplantation etc.), asthma, abnormal bone formation, neuronal cell death, hepatopathy, abortion, premature birth or retina neuropathy of glaucoma etc.
- immunological diseases autoimmune diseases, organ transplantation etc.
- asthma abnormal bone formation
- neuronal cell death hepatopathy
- abortion premature birth or retina neuropathy of glaucoma etc.
- the compounds of formula (I), non-toxic salts thereof, cyclodextrin clathrates thereof may be normally administered systemically or partially, by oral or parenteral administration.
- the doses to be administered are determined depending upon age, body weight, symptom, the desired therapeutic effect, the route of administration, and the duration of the treatment etc.
- the doses per person per dose are generally from 1 ⁇ g to 100 mg, by oral administration, from once up to several times per day, and from 0.1 ⁇ g to 10 mg, by parenteral administration (preferred into vein) from once up to several times per day, or by continuous administration for from 1 hour to 24 hours per day into vein.
- the doses to be used depend upon various conditions. Therefore, there are cases in which doses lower than or greater than the ranges specified above may be used.
- the compounds of the present invention may be administered in the form, for example, solidcompositions, liquidcompositions or other compositions for oral administration, or injections, liniments or suppositories etc. for parenteral administration.
- Solid compositions for oral administration include compressed tablets, pills, capsules, dispersible powders, and granules.
- Capsules include hard capsules and soft capsules.
- one or more of the active compound(s) are formulated by conventional method as it is or after admixed with excipients (lactose, mannitol, glucose, microcrystalline cellulose, starch etc.), connecting agents (hydroxypropyl cellulose, polyvinyl pyrrolidone, magnesium metasilicate aluminate etc.), disintegrating agents (cellulose calcium glycolate etc.). lubricating agents (magnesiumstearateetc.), stabilizing agents, agents to assist dissolution (glutamic acid, aspartic acid etc.) etc.
- excipients lactose, mannitol, glucose, microcrystalline cellulose, starch etc.
- connecting agents hydroxypropyl cellulose, polyvinyl pyrrolidone, magnesium metasilicate aluminate etc.
- disintegrating agents cellulose calcium glycolate etc.
- lubricating agents magnesiumstearateetc.
- stabilizing agents agents to assist dissolution (glutamic acid, aspartic acid etc.) etc.
- the tablets or pills may, if desired, be coated with coating agents (sugar, gelatin, hydroxypropyl cellulose, hydroxypropyl methyl cellulose phthalate etc.), or be coated with more than one films. Coating may include containment within capsules of absorbable materials such as gelatin.
- Liquid compositions for oral administration include pharmaceutically acceptable aqueous solutions, suspensions, emulsions, syrups and elixirs etc.
- one or more of the active compound(s) may be contained in diluent(s) commonly used in the art (purified water, ethanol or a mixture thereof etc.).
- diluent(s) commonly used in the art (purified water, ethanol or a mixture thereof etc.).
- These liquid compositions may also comprise wetting agents, suspending agents, emulsifying agents, sweetening agents, flavoring agents, perfuming agents and preserving agents, buffering agents etc.
- Injections for parenteral administration include solutions, suspensions and solid injections which may be dissolved or suspended in solvents before use. Injections are prepared by dissolving, suspending or emulsifying one or more of the active compound(s) in solvents.
- Solvents include, for example, distilled water for injection and physiological salt solution, vegetable oil, propylene glycol, polyethylene glycol, alcohol such as ethanol and the combination thereof.
- These injections may include stabilizing agents, agents to assist dissolution (glutamic acid, aspartic acid, POLYSORBATE8O (registered trade mark) etc.), suspending agents, emulsifying agents, pain-removing agents, buffering agents, preserving agents etc. These are sterilized in the final scheme or are prepared by asepticism.
- Sterile solid compositions such as freeze-dried composition, may be prepared, to sterilize or to solve in sterile distilled water for injection or other sterile solvents before use.
- compositions for parenteral administration include liquids for external use, and ointment, endermic liniments, nhalants, spray compositions, suppositories for rectal dministration and pessaries for vaginal administration etc. hich comprise one or more of the active compound(s) and may be prepared by conventional methods.
- Spray compositions may comprise stabilizing agents such as sodium sulfite hydride, isotonic buffers such as sodium chloride, sodium citrate or citric acid).
- stabilizing agents such as sodium sulfite hydride, isotonic buffers such as sodium chloride, sodium citrate or citric acid.
- the solvents in parentheses show the developing or eluting solvents and the ratios of the solvents used are by volume in chromatographic separations.
- the solvents in parentheses in NMR show the solvents used in measurement.
- TBS is t-butyldimethylsilyl.
- cuprous cyanide (896 mg) and lithium chloride (890 mg) were dissolved in THF (10 ml), and the mixture was stirred for 3 hours.
- the organic layer was washed with water twice, with a saturated aqueous solution of sodium chloride once, and concentrated.
- the residue was dissolved in methanol/ether (2 ml+1 ml) mixed solvents, and the mixture was stirred at room temperature.
- Pyridinium p-toluenesulfonate (4 mg) was added therein, and the mixture was stirred at room temperature for 1 hour.
- the reaction was terminated by addition of a saturated aqueous solution of sodiumbicarbonate, and extracted with ethyl acetate three times.
- the organic layer was washed with water twice, with a saturated aqueous solution of sodium chloride once, and concentrated.
- the following compounds were admixed in the conventional method, dried, and was added microcrystalline cellulose thereon to weigh 10 g in total, mixed until homogeneous and punched out in conventional method to obtain 100 tablets each containing 30 ⁇ g of active ingredient.
Abstract
(wherein all symbols are as defined in the description);
and non-toxic salts thereof, prodrugs thereof and cyclodextrin clathrates thereof.
Compounds of formula (I) strongly bind on the EP2 subtype receptor. Therefore, they are useful for prevention and/or treatment of immunological diseases (autoimmune diseases, organ transplantation etc.), asthma, abnornmal bone formation, neuronal cell death, liver damage, abortion, premature birth or retina neuropathy of glaucoma etc.
Description
This invention relates to ω-cycloalkyl-prostaglandin E2 derivatives, a process for the preparation thereof, and a pharmaceutical agent containing it as an active ingredient. More particularly, this invention relates to
(wherein all symbols are as defined hereinafter)
or non-toxic salts thereof, or prodrugs thereof or cyclodextrin clathrates thereof,
(2) processes for the preparation thereof, and
(3) a pharmaceutical agent containing it as an active ingredient.
Prostaglandin E2 (abbreviated as PGE2 hereinafter) has been known as a metabolite in the arachidonic acid cascade. It has been known that PGE2 has cyto-protective activity, uterine contractile activity, a pain-inducing effect, a promoting effect of digestive peristalsis, an awakening effect, a suppressive effect of gastric acid secretion, hypotensive activity and diuretic activity etc.
In the recent study, it was found that PGE2 receptor was divided into some subtypes which possess different physiological roles each other. At present, four receptor subtypes are known and they are called EP1, EP2, EP3 and EP4 (Negishi M. et al., J. Lipid Mediators Cell Signaling, 12, 379-391 (1995)).
The present inventors investigated to find new compounds which bind on each receptor specifically, so that we found that the compounds of the present invention could bind strongly on EP2 subtype receptor and achieved the present invention.
The compounds of the present invention of formula (I), possess a strong binding activity on EP2 subtype receptor. Therefore, they are useful for prevention and/or treatment of immunological diseases (autoimmune diseases, organ transplantation etc.), asthma, abnormal bone formation, neuronal cell death, hepatopathy, abortion, premature birth or retina neuropathy of glaucoma etc.
Among the compounds of the present invention of formula (I), compounds which bind weakly on receptor subtypes except EP2 receptor and on other receptors for arachidonic acid cascade metabolites (thromboxane receptor, PGI2 receptor etc.) do not express other effects and therefore, it is probable that these compounds will be medical agents which have less side-effects.
On the other hand, many patent applications of PG derivatives are known. The following application is mentioned for example.
[wherein R1A and R2A are hydrogen; R3A is hydrogen or is taken together with R4A to form a methylene chain of 4 carbon atoms wherein a cycloalkyl of 6 carbon atoms inclusive is formed, or is taken together with R4A to form a bicycloalkenyl or bicycloalkyl moiety having the formula
(wherein pA is an integer having a value of from 0 to 1 and qA is an integer having a value of from 2 to 3 and wherein the double bond of such bicycloalkenyl is in the qA bridge); R4A together with R3A forms a cycloalkyl, bicycloalkyl or bicycloalkenyl as defined above, or together with R5A forms a methylene chain of 3 carbon atoms wherein a cycloalkyl of 4 carbon atoms inclusive is formed; R5A is hydrogen, or is taken together with R to form a cycloalkyl as defined above; and R6A is hydrogen or straight-chain alkyl having 8 carbon atoms.]
are disclosed as having prostaglandin-like activity.
The present invention relates to
(wherein A is benzene, thiophene or furan ring;
R1 is hydroxy, C1-6 alkoxy or a group of formula
(wherein R10 and R11 are each independently, hydrogen atom or C1-4 alkyl);
R2 is C1-4 alkylene, C2-4 alkenylene, —S—C1-4 alkylene, —S—C2-4 alkenylene or C1-4 alkylene-S—;
R3 is oxo, methylene, halogen atom or a group of formula
(wherein R32 is C1-4 alkyl, C1-4 alkoxy, phenyl, phenyl-C1-4 alkyl,
R33—OOC—C1-4 alkyl or R33—OOC—C2-4 alkenyl (wherein R33 is hydrogen atom or C1-4 alkyl);
R4 is hydrogen atom, hydroxy or C1-4 alkoxy;
R5 is C1-8 alkyl, C2-8 alkenyl, C2-8 alkynyl, or C1-8 alkyl, C2-8 alkenyl or C2-8 alkynyl substituted by 1-3 substituents selected from (1)-(5) below:
(1) halogen atom,
(2) C1-4 alkoxy,
(3) C3-7 cycloalkyl,
(4) phenyl,
(5) phenyl substituted by 1-3 substituents selected from halogen atom, C1-4 alkyl, C1-4 alkoxy, nitro or trifluoromethyl;
n is 0-4;
is single bond or double bond;
with the proviso that when the C8-9 position is double bond, R3 is R32—COO—, and R1 is C1-6 alkoxy),
a non-toxic salt thereof, a prodrug thereof or a cyclodextrin clathrate thereof,
(2) a process for the preparation thereof, and
(3) a pharmaceutical agent containing it as an active ingredient.
In formula (I), C1-4 alkyl in the definitions of R11, R12, R32, R33 and R5 means methyl, ethyl, propyl, butyl and isomers thereof.
In formula (I), C1-8 alkyl in the definitions of Rs means methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl and isomers thereof.
In formula (I), C1-4 alkoxy represented by R32, R4and Rs means methoxy, ethoxy, propoxy, butoxy and isomers thereof.
In formula (I), C1-6 alkoxy representedby R′ means methoxy, ethoxy, propoxy, butoxy, pentyloxy, hexyloxy and isomers thereof.
In formula (I), C2-4 alkenylin the definitions of R32means vinyl, propenyl, butenyl and isomers thereof.
In formula (I), C1-4 alkylene represented by R2is methylene, dimethylene, trimethylene, tetramethylene and isomers thereof.
In formula (I), C2-4 alkylenerepresentedby R2is vinylene, propenylene, butenylene and isomers thereof.
In formula (I), C2-8 alkenyl represented by R3 means vinyl, propenyl, butenyl, pentenyl, hexenyl, heptenyl, octenyl and isomers thereof.
In formula (I), C2-8 alkynyl representedby R5 means ethynyl, propynyl, butynyl, pentynyl, hexynyl, heptynyl, octynyl and isomers thereof.
In formula (I), C3-7 cycloalkyl in the definitions of R5 means cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl and cycloheptyl.
In formula (I), halogen atom in the definitions of R3 and R5 means fluorine, chlorine, bromine and iodine.
In the present invention, it may be easily understood by those skilled in the art, unless otherwise specified, the symbol:
indicates that the substituent attached thereto is in front of the sheet, unless otherwise specified, the symbol:
indicates that the substituent attached thereto is behind the sheet,
unless otherwise specified, the symbol:
indicates that the substituent attached thereto may be in front of or behind the sheet or may be a mixture of isomers in front of and behind the sheet.
Unless otherwise specified, all isomers are included in the present invention. For example, the alkyl, alkenyl and alkynyl groups include straight-chain and also branched-chain ones. The double bond in alkenyl group includes E, Z and EZ mixed isomers. Isomers resulting from the presence of asymmetric carbon atom(s) e.g. in branched-chain alkyl are included in the present invention.
In the present invention, in the case of a compound wherein the C7 position is sulfur, the configuration of C8 position of the compounds of the present invention are shown as 8α, but as is known in the art, these 8α-compounds are in the equilibrium state with 8β-compounds (8-epi compound). Therefore the compounds of formula (I) mean mixtures of 8α-compound and isomeric 8β-compound.
Preferred compounds of the present invention include compounds of the formula (I) listed in the examples or in Tables 1-20 below.
| TABLE 1 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 2 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 3 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 4 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 5 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 6 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 7 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 8 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 9 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 10 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 11 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 12 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 13 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 14 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 15 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 16 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 17 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 18 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 19 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
| TABLE 20 |
|
|
| No. | n | R5 |
| 1 | 0 |
|
| 2 | 0 |
|
| 3 | 0 |
|
| 4 | 0 |
|
| 5 | 0 |
|
| 6 | 0 |
|
| 7 | 0 |
|
| 8 | 0 |
|
| 9 | 0 |
|
| 10 | 0 |
|
| 11 | 1 |
|
| 12 | 1 |
|
| 13 | 1 |
|
| 14 | 1 |
|
| 15 | 1 |
|
| 16 | 1 |
|
| 17 | 1 |
|
| 18 | 1 |
|
| 19 | 1 |
|
| 20 | 1 |
|
[Salts]
The compounds of the present invention of formula (I) may be converted into the corresponding salts by a conventional means. Non-toxic, and water-soluble salts are preferable. Appropriate salts are described below; salts of alkali metals (potassium, sodium etc.), salts of alkaline-earth metals (calcium, magnesium etc.), ammonium salts, salts of pharmaceutically acceptable organic amines (tetramethyl ammonium, triethylamine, methylamine, dimethylamine, cyclopentylamine, benzylamine, phenethylamine, piperidine, monoethanolamine, diethanolamine, tris(hydroxymethyl)methylamine, lysine, arginine, N-methyl-D-glucamine etc.).
[Cyclodextrin clathrates]
Prostanic acid derivatives of formula (I) may be converted into cyclodextrin clathrates using α-, β- or γ-cyclodextrin or a mixture thereof, by the methods described in the specification of Japanese Kokoku No.50-3363 or Japanese Kokoku No.52-31404 (i.e. GB Patent Nos. 1351238 or 1419221). Converting into their cyclodextrin clathrates serves to increase the stability and solubility in water of the compounds, and therefore it is useful in the use for pharmaceuticals.
(1) Among the compounds of the present invention of formula (I), a compound (wherein R3 is oxo, methylene or halogen atom; R1 is hydroxy); i.e. a compound of formula (IA)
wherein R30 is oxo, methylene or halogen atom, and the other symbols are as defined above)
may be prepared by subjecting to hydrolysis using an enzyme or hydrolysis under alkaline conditions a compound of formula (IB)
(wherein R12 is C1-6 alkyl, and the other symbols are as defined above).
Hydrolysis using an enzyme is known, for example, it may be carried out in a mixture of a water-miscible organic solvent (ethanol, dimethylsulfoxide etc.) and water, in the presence or absence of buffer, using an ester-cleaving enzyme (esterase, lipase etc.) at a temperature of from 0° C. to 50° C.
Hydrolysis under alkaline conditions is known, for example, it may be carried out in awater-miscible organic solvent (ethanol, tetrahydrofuran (THF), dioxane etc.) using an aqueous solution of an alkali (sodium hydroxide, potassium hydroxide, potassium carbonate etc.) at a temperature of from −10° C. to 90° C.
(2) Among the compounds of the present invention of formula (I), a compound (wherein R3 is oxo, methylene or halogen atom; R1 is a group of formula
(wherein all symbols are as defined above)
(wherein all symbols are as defined above)
with a compound of formula (II)
(wherein all symbols are as defined above).
Amidation is known, for example, it may be carried out in an inert organic solvent (THF, methylene chloride, benzene, acetone, acetonitrile or a mixture thereof etc.), in the presence or absence of a tertiary amine (dimethylaminopyridine, pyridine, triethylamine etc.), using a condensing agent (1,3-dicyclohexylcarbodiimide (DCC), 1-ethyl-3-[3-(dimethylamino) propyl]carbodiimide (EDC) etc.) at a temperature of from 0° C. to 50° C.
(3) Among the compounds of formula (IB), a compound (wherein R4 is hydrogen atom or hydroxy); i.e. a compound of formula (IB-1)
(wherein R40 is hydrogen atom or hydroxy, and the other symbols are as defined above)
(wherein R41 is hydrogen atom or hydroxy protected by a protective group which may be removed under acidic conditions; R60 is a protective group of hydroxy which may be removed under acidic conditions, and the other symbols are as defined above.)
A protective group of hydroxy which may be removed under acidic conditions includes, for example, t-butyldimethylsilyl, triphenylmethyl, tetrahydropyran-2-yl etc.
Hydrolysis under acidic conditions is known; for example, it may be carried out in a water-miscible organic solvent (tetrahydrofuran, methanol, ethanol, dimethoxyethane, acetonitrile or a mixture thereof etc.) using an inorganic acid (hydrochloric acid, phosphoric acid, hydrofluoric acid, hydrofluoric acid-pyridine complex etc.) or an organic acid (acetic acid, toluenesulfonic acid, trichloroacetic acid etc.), at a temperature of from 0° C. to 50° C.
(4) Among the compounds of formula (IB), a compound
(wherein R 42 is C1-4 alkoxy and the other symbols are as defined above) may be prepared by O-alkylation of a compound of formula (IB) wherein R4 is hydroxy, i.e. a compound of formula (IB-3)
(wherein all symbols are as defined above).
O-Alkylation is known, for example, it may be carried out in an inert organic solvent (THF, diethyl ether etc.), using diazoalkane at a temperature of from −30° C. to 40° C. or in an inert organic solvent (acetonitrile etc.), in the presence of silver oxide, using alkyl iodide at a temperature of from 0° C. to 40° C.
(5) Among the compounds of formula (I) wherein R3 is a group of formula
(wherein all symbols are as defined above)
(wherein all symbols are as defined above).
Deprotection reaction may be carried out as described above.
A compound of formula (IV) may be prepared by scheme (G) or (K) described below.
A compound of formula (III) may be divided into the following six classes of compounds according to the values of R30 and R41. That is,
1) R30 is oxo and R41 is hydroxy protected by a protective group which may be removed under acidic conditions, i.e. a compound of formula (IIIA)
wherein all symbols are as defined above).
2) R30 is methylene and R41 is hydroxy protected by a protective group which may be removed under acidic conditions, i.e. a compound of formula (IIIB)
(wherein all symbols are as defined above).
3) R30 is halogen atom and R41 is hydroxy protected by a protective group which may be removable under acidic conditions, i.e. a compound of formula (IIIC)
(wherein R31 is halogen atom, and the other symbols are as defined above).
(wherein all symbols are as defined above).
(wherein all symbols are as defined above).
(wherein all symbols are as defined above).
A compound of formula (IIIB) may be prepared from a compound of formula (IIIA) according to the reaction of the following Scheme (A).
A compound of formula (IIIC) may be prepared from a compound of formula (IIIA) according to the reactions of the following Scheme (B), (C) or (D).
A compound of formula (IIID) may be prepared from a compound of formula (IIA) according to the reactions of the following Scheme (E).
A compound of formula (IIIE) may be prepared from a compound of formula (IIID) according to the same reactions as the following Scheme (A).
A compound of formula (IIIF) may be prepared from a compound of formula (IIID) according to the same reactions as the following Scheme (B), (C) or (D).
A compound of formula (IIIA) may be prepared according to the reactions of the following Scheme (F), (G), (H) or (J).
In the reaction Schemes, the symbols represent the following meanings or are as described above.
R21 is C1-3 alkylene or C2-3 alkenylene;
R22 is C1-3 alkylene;
t-Bu is t-butyl;
n-Bu is normal butyl;
c-Hex is cyclohexyl;
Et is ethyl;
EE is ethoxyethyl;
Ac is acetyl;
Ph is phenyl;
Ts is p-toluenesulfonyl;
Ms is methanesulfonyl;
DMAP is dimethylaminopyridine;
AIBN is 2,2′-azobisisobutyronitrile;
Each reaction in reaction Schemes described above is carried out by known methods. In the reaction Schemes described above, the compounds of formula (V), (VII), (VIII), (IX), (X), (XII), (XIII) and (XIV) as starting materials are known per se or may be prepared by known methods.
For example, among the compounds of formula (V), (4RS) -5,5-propanooct-1-yn-4-ol is a known compound described in the specification of JP54-115351.
Among the compounds of formula (X), (4R)-2-(diethylaminomethyl)-4-t-butyldimethylsilyloxy-2-cyclopenten-1-one is a known compound described in J. Org. Chem., 53, 5590-5592 (1988).
Among the compounds of formula (VIII), (4R)-4-t-butyldimethylsilyloxy-2-cyclopenten-1-one is a known compound described in J. Am. Chem. Soc., 110, No. 14, 4718-4726 (1988).
The other starting materials and reagents in the present invention are known per se or may be prepared by known methods.
In each reaction in the present specification, obtained products may be purified by conventional techniques. For example, purification maybe carried out by distillation under atmospheric or reduced pressure, by high performance liquid chromatography, by thin layer chromatography or by column chromatography using silica gel or magnesium silicate, by washing or by recrystallization. Purification may be carried out after each reaction, or after a series of reactions.
[Pharmacological Activities]
The compounds of the present invention of formula (I) bind and act on EP2 receptor which is a subtype of PGE2 receptor.
For example, the effects of the compounds of the present invention were confirmed by binding assay using expression cell of prostanoids receptor subtype.
(i) Binding Assay Using Expression Cell of Prostanoids Receptor Subtype
The preparation of membrane fraction was carried out according to the method of Sugimoto et. al., [J. Biol. Chem., 267, 6463-6466 (1992)], using expression CHO cell of the prostanoids receptor subtype (mouse EP1, EP2, EP3α, EP4).
The standard assay mixture containing membrane fraction (0.5 mg/ml) and [3H]-PGE2 (2.5 nm) in a final volume of 200 μl was incubated at room temperature for 1 hour. The reaction was terminated by addition of ice-cooled buffer (3 ml). The mixture was filtered through a GF/B glass filter under reduced pressure. The radioactivity associated with the filter was measured by liquid scintillation counter.
Kd and Bmax values were determined from Scatchard plots [Ann. N. Y. Acad. Sci., 51, 660 (1949)]. Non-specific binding was calculated as the binding in the presence of an excess (2.5 μM) of unlabeled PGE2. In the experiment for competition of specific 3H-PGE2binding by the compounds of the present invention, 2.5 nM of 3H-PGE2 and various concentrations of compounds of the present invention were added. The following buffer was used in all reactions.
Buffer: 10 mM potassium phosphate (pH 6.0), 1 mM EDTA, 10 mM MgCl2, 0.1 M NaCl.
All the values shown are those obtained using the more polar stereoisomer of the exemplified compounds. The dissociation constant; i.e. Ki (μM) of each compound was calculated by the following equation.
The results are shown in Table 21.
| TABLE 21 | ||
| Example | Ki (μM) | |
| No. | EP1 | EP2 | EP3α | EP4 |
| 2(a) | >10 | 0.0097 | >10 | >10 |
| 2(b) | >10 | 0.0061 | >10 | >10 |
| 5(b) | >10 | 0.019 | >10 | 1.50 |
| 5(d) | >10 | 0.002 | >10 | 0.31 |
| 9(c) | >10 | 0.0051 | >10 | >10 |
[Toxicity]
The toxicity of the compounds of the present invention is very low and therefore, it is confirmed that these compounds are safe for pharmaceutical use.
The compounds of the present invention of formula (I) bind strongly and act on PGE2 receptor, especially on EP2 subtype receptor and therefore are useful for prevention and/or treatment of immunological diseases (autoimmune diseases, organ transplantation etc.), asthma, abnormal bone formation, neuronal cell death, hepatopathy, abortion, premature birth or retina neuropathy of glaucoma etc.
Among the compounds of the present invention of formula (I), compounds which bind weakly on receptor subtypes except EP2 receptor and on other receptors for arachidonic acid cascade metabolites (thromboxane receptor, PGI2 receptor etc.) do not express other effects and therefore it is probable that these compounds will be medical agents which have less side-effects.
For the purpose described above, the compounds of formula (I), non-toxic salts thereof, cyclodextrin clathrates thereof may be normally administered systemically or partially, by oral or parenteral administration.
The doses to be administered are determined depending upon age, body weight, symptom, the desired therapeutic effect, the route of administration, and the duration of the treatment etc. In the human adult, the doses per person per dose are generally from 1 μg to 100 mg, by oral administration, from once up to several times per day, and from 0.1 μg to 10 mg, by parenteral administration (preferred into vein) from once up to several times per day, or by continuous administration for from 1 hour to 24 hours per day into vein.
As mentioned above, the doses to be used depend upon various conditions. Therefore, there are cases in which doses lower than or greater than the ranges specified above may be used.
The compounds of the present invention may be administered in the form, for example, solidcompositions, liquidcompositions or other compositions for oral administration, or injections, liniments or suppositories etc. for parenteral administration.
Solid compositions for oral administration include compressed tablets, pills, capsules, dispersible powders, and granules. Capsules include hard capsules and soft capsules.
In these solid compositions for oral administration, one or more of the active compound(s) are formulated by conventional method as it is or after admixed with excipients (lactose, mannitol, glucose, microcrystalline cellulose, starch etc.), connecting agents (hydroxypropyl cellulose, polyvinyl pyrrolidone, magnesium metasilicate aluminate etc.), disintegrating agents (cellulose calcium glycolate etc.). lubricating agents (magnesiumstearateetc.), stabilizing agents, agents to assist dissolution (glutamic acid, aspartic acid etc.) etc. The tablets or pills may, if desired, be coated with coating agents (sugar, gelatin, hydroxypropyl cellulose, hydroxypropyl methyl cellulose phthalate etc.), or be coated with more than one films. Coating may include containment within capsules of absorbable materials such as gelatin.
Liquid compositions for oral administration include pharmaceutically acceptable aqueous solutions, suspensions, emulsions, syrups and elixirs etc. In such liquid compositions, one or more of the active compound(s) may be contained in diluent(s) commonly used in the art (purified water, ethanol or a mixture thereof etc.). These liquid compositions may also comprise wetting agents, suspending agents, emulsifying agents, sweetening agents, flavoring agents, perfuming agents and preserving agents, buffering agents etc.
Injections for parenteral administration include solutions, suspensions and solid injections which may be dissolved or suspended in solvents before use. Injections are prepared by dissolving, suspending or emulsifying one or more of the active compound(s) in solvents. Solvents include, for example, distilled water for injection and physiological salt solution, vegetable oil, propylene glycol, polyethylene glycol, alcohol such as ethanol and the combination thereof. These injections may include stabilizing agents, agents to assist dissolution (glutamic acid, aspartic acid, POLYSORBATE8O (registered trade mark) etc.), suspending agents, emulsifying agents, pain-removing agents, buffering agents, preserving agents etc. These are sterilized in the final scheme or are prepared by asepticism. Sterile solid compositions, such as freeze-dried composition, may be prepared, to sterilize or to solve in sterile distilled water for injection or other sterile solvents before use.
Other compositions for parenteral administration include liquids for external use, and ointment, endermic liniments, nhalants, spray compositions, suppositories for rectal dministration and pessaries for vaginal administration etc. hich comprise one or more of the active compound(s) and may be prepared by conventional methods.
Spray compositions may comprise stabilizing agents such as sodium sulfite hydride, isotonic buffers such as sodium chloride, sodium citrate or citric acid). The preparation of spray compositions, for example, is described in the U.S. Pat. No. 2,868,691 or No. 3,095,355 in detail.
The following reference examples and examples are intended to illustrate, but do not limit, the present invention.
The solvents in parentheses show the developing or eluting solvents and the ratios of the solvents used are by volume in chromatographic separations. The solvents in parentheses in NMR show the solvents used in measurement. In the examples, TBS is t-butyldimethylsilyl.
To the mixture solution of (4RS)-5,5-propanooct-1-yn-4-ol (4.0 g) and imidazole (4.9 g) in dimethylformamide (50 ml) was added t-butyldimethylsilyl chloride (5.4 g) under cooling with ice. The reaction mixture was stirred at 60° C. for 7 hours. The reaction was quenched by addition of water. The mixture was extracted with ethyl acetate. The extract was washed with water and a saturated aqueous solution of sodium chloride, dried over anhydrous magnesium sulfate and concentrated. The residue was purified by column chromatography on silica gel (hexane→hexane:ethyl acetate=10:1) to give the title compound (6.8 g) having the following physical data.
TLC: Rf 0.64 (hexane);
NMR (CDCl3): δ 3.75 (1H, t, J=5.8 Hz), 2.28 (1H, ddd, J=17, 5.0, 2.5 Hz), 2.16 (1H, ddd, J=17, 6.0, 2.5 Hz), 2.10-1.94 (1H, m), 1.92 (1H, t, J=2.5 Hz), 1.90-1.20 (9H, m), 0.90 (3H, t, J=6.0 Hz), 0.89 (9H, s), 0.12 (3H, s), 0.07 (3H, s)
To the mixture of the compound prepared in reference example 1 (3.0 g) and tributyl tinhydride (3.7 ml) was added azobisisobutyronitrile (35 mg). The mixture was stirred at 80° C. for 1.5 hours. After the mixture was cooled to room temperature, to the mixture was added dropwise iodine (4.1 g) in methylene chloride (70 ml). The reaction mixture was stirred for 10 min. To the reaction mixture were added a saturated solution of sodium thiosulfate, ethyl acetate and a saturated aqueous solution of sodium chloride. The mixture was stirred, filtered, and the aqueous layer was extractedwith ethyl acetate. The organic layer was washed with a saturated aqueous solution of sodium chloride, dried over anhydrous magnesium sulfate and concentrated. The residue was purified by column chromatography on silica gel (hexane) to give the title compound (3.9 g) having the following physical data.
TLC: Rf 0.77 (hexane);
NMR (CDCl3): δ 6.49 (1H, dt, J=14.5, 7.5 Hz), 5.97 (1H, d, J=14.5 Hz), 3.58 (1H, t, J=6.0 Hz), 2.20-1.20 (12H, m), 0.91 (3H, t, J=6.0 Hz), 0.91 (9H, s), 0.06 (3H, s), 0.05 (3H, s).
(3S,4R)-t-Butyldimethylsilyloxy-2-methylene-3-(4-t-butyldimethylsilyloxy-5,5-propanooct-1-enyl)cyclopentan-1-one
Under atmosphere of argon, to a stirred solution of (1E,4RS)-1-iodo-4-t-butyldimethylsilyloxy-5,5-propanooct-1-ene (302 mg, which was prepared in reference example 2) in diethyl ether (5 ml) at −78° C., was added t-butyllithium/pentane solution (0.87 ml, 1.64 M) dropwise, and the mixture was stirred for 45 minutes. To the mixture was added dropwise 2-thienyllithium cyanocuprate/THF solution (3.6 ml, 0.25 M) and the mixture was stirred for 20 minutes. To the solution was added (4R)-4-t-butyldimethylsilyloxy-2-diethylamino methylcyclopent-2-en-1-one (200 mg) dissolved in diethyl ether (3 ml), and the mixture was stirred warming from −78° C. to 0° C. for 1 hour. The reaction was terminated by addition of a saturated solution of ammonium chloride, and the mixture was extracted with ethyl acetate three times. The organic layer was washed with a saturated solution of ammonium chloride twice, a saturated solution of sodium chloride once, dried over anhydrous magnesium sulfate. The residue was purified by column chromatography on silica gel (Fuji Silysia BW-300 40 ml, ethyl acetate/hexane=0/1→1/50) to give the title compound (235 mg) having the following physical data as a sepia oil.
TLC: Rf 0.54 (ethyl acetate:hexane=1:15).
(11α, 13E)-9-Oxo-11,15-bis(t-butyldimethylsilyloxy)-17,17-propano-1,6- (p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
A. Preparation of Reformatosky Reagent
Under atmosphere of argon, to a suspension of zinc powder (654 mg) stirred in THF (0.5 ml) at room temperature, was added dropwise dibromoethane (15 ml), and the mixture was stirred at 60° C. for 2 minutes. The reaction mixture was cooled to room temperature, and trimethylsilyl chloride (22 μl) was added dropwise therein, and the mixture was stirred at 0° C. A solution of benzylbromide (1.14 g) in THF (4.5 ml) was added therein, and the mixture was stirred at 10° C. for 3 hours.
B. Preparation for cuprous reagent
Under atmosphere of argon, cuprous cyanide (896 mg) and lithium chloride (890 mg) were dissolved in THF (10 ml), and the mixture was stirred for 3 hours.
C. Michael Addition Reaction
Under atmosphere of argon, to a Reformatosky reagent (0.58 ml) stirred at −78° C., was added dropwise the cuprous reagent (0.72 ml). The reaction solution was stirred at −78° C. for 30minutes. Trimethylsilylchloride (66 μl) andthecompound prepared in reference example 3 (150 mg) in THF (2 ml) were added dropwise therein successively. The reaction solution was stirred at −78° C. for 1 hour and at −20° C. for 2 hours. The reaction was terminated by addition of a saturated solution of ammonium chloride, and the mixture was extracted with ethyl acetate three times. The organic layer was washed with water twice, with a saturated aqueous solution of sodium chloride once, and concentrated. The residue was dissolved in methanol/ether (2 ml+1 ml) mixed solvents, and the mixture was stirred at room temperature. Pyridinium p-toluenesulfonate (4 mg) was added therein, and the mixture was stirred at room temperature for 1 hour. The reaction was terminated by addition of a saturated aqueous solution of sodiumbicarbonate, and extracted with ethyl acetate three times. The organic layer was washed with water twice, with a saturated aqueous solution of sodium chloride once, and concentrated. The residue was purified by column chromatography on silica gel (Merck 7734 20 ml, ethyl acetate/hexane=1/20→1/5) to give the title compound (53 mg) having the following physical data as a colorless oil.
TLC: Rf 0.60 (ethyl acetate:hexane=1:5);
NMR (CDCl3): δ 7.94 (2H, d, J=8.5 Hz), 7.23 (2H, d, J=8.5 Hz), 5.80-5.50 (1H, m), 5.40-5.20 (1H, m), 4.96 (1H, dd, J=7.5, 2.5 Hz), 3.70 (3H, s), 3.57 (1H, t, J=5.0 Hz), 2.90-1.10 (20H, m), 1.00-0.75 (21H, m), 0.10-0.00 (6H, m).
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
Under atmosphere of argon, to a solution of the compound prepared in reference example 4 (53 mg) in acetonitrile (2 ml) stirred at 0° C., was added an aqueous solution of hydrofluoric acid (0.2 ml, 48%), and the mixture was stirred for 3 hours. The reaction was terminated by addition of water, and the solution was extracted with ethyl acetate three times. The organic layer was washed with water twice, with a saturated aqueous solution of sodium chloride once, and concentrated. The residue was purified by column chromatography on silica gel (Merck Lobar prepacked column size A, ethyl acetate/hexane=1/1) to give the title compounds (less polar isomer (12 mg) and more polar isomer (11.5 mg)) having the following physical data as a colorless oil respectively.
Less Polar
TLC: Rf 0.28 (ethyl acetate:hexane=1:1);
NMR (CDCl3): δ 7.94 (2H, d, J=8.5 Hz), 7.24 (2H, d, J=8.5 Hz), 5.74 (1H, dt, J=15.5, 7.0 Hz), 5.43 (1H, dd, J=15.5, 8.5 Hz), 4.06 (1H, q, J=9.0 Hz), 3.90 (3H, s), 3.58 (1H, dd, J=9.5, 3.0 Hz), 3.00-2.80 (3H, m), 2.50-1.20 (19H, m), 0.95 (3H, t, J=6.5 Hz).
More Polar
TLC: Rf 0.22 (ethyl acetate:hexane=1:1);
NMR (CDCl3): δ 7.94 (2H, d, J=8.5 Hz), 7.24 (2H, d, J=8.5 Hz), 5.69 (1H, ddd, J=15.0, 9.0, 5.0 Hz), 5.37 (1H, dd, J=15.0, 9.5 Hz), 4.03 (1H, q, J=8.0 Hz), 3.90(3H, S), 3.56 (1H, dd, J=10.0, 2.5 Hz), 3.00-2.60 (3H, m), 2.50-1.20 (19H, m), 0.94 (3H, t, J=7.0 Hz).
By the same procedure as reference example 1, 2, 3, 4 and example 1, the compounds having the following physical data were obtained.
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13,19-dienoic Acid Methyl Ester
Less Polar
TLC: Rf 0.42 (hexane:ethyl acetate=1:2);
NMR (CDCl3): δ 7.94 (2H, d, J=8.2 Hz), 7.25 (2H, d, J=8.2 Hz), 5.96 (1H, ddt, J=17.0, 10.0, 7.2 Hz), 5.74 (1H, dt, J=15.4, 6.8 Hz), 5.42 (1H, dd, J=15.4, 8.6 Hz), 5.20-5.05 (2H, m), 4.15-3.98 (1H, m), 3.90 (3H, s), 3.59 (1H, dd, J=9.5, 2.7 Hz), 2.90-2.65 (3H, m), 2.60-1.60 (17H, m).
More Polar
TLC: Rf 0.36 (hexane:ethyl acetate=1:2);
NMR (CDCl3): δ 7.94 (2H, d, J=8.2 Hz), 7.24 (2H, d, J=8.2 Hz), 5.95 (1H, ddt, J=17.0, 10.0, 7.2 Hz), 5.69 (1H, ddd, J=15.0, 8.6, 5.4 Hz), 5.37 (1H, dd, J=15.0, 8.6 Hz), 5.20-5.05(2H, m), 4.10-3.95 (1H, m), 3.90 (3H, s), 3.57 (1H, dd, J=10.2, 2.2 Hz), 2.90-2.65 (3H, m), 2.45-1.55 (17H, m).
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-19,20 -methano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
More Polar
TLC: Rf 0.56 (hexane ethyl:acetate=1:2);
NMR (CDCl3): δ 7.94 (2H, d, J=8.3 Hz), 7.24 (2H, d, J=8.3 Hz), 5.69 (1H, ddd, J=15, 9.1, 5.5 Hz), 5.36 (1H, dd, J=15, 8.7 Hz), 4.02 (1H, m), 3.90 (3H, s), 3.69 (1H, dd, J=10, 1.4 Hz), 2.78 (3H, m), 2.56-1.63 (13H, m), 1.51 (1H, dd, J=14, 6.6 Hz), 1.38 (1H, dd, J=14, 6.2 Hz), 0.77 (1H, m), 0.50 (2H, m), 0.11 (2H, m).
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-19-methyl-1,6 -(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
More Polar
TLC: Rf 0.64 (hexane:ethyl acetate=1:2);
NMR (CDCl3): δ 7.94 (2H, d, J=8.2 Hz), 7.24 (2H, d, J=8.2 Hz). 5.69 (1H, ddd, J=15, 9.4, 5.3 Hz), 5.38 (1H, dd, J=15, 8.7 Hz), 4.03 (1H, m), 3.90 (3H, s), 3.64 (1H, dd, J=10, 1.5 Hz), 2.77 (3H, m), 2.58-1.63 (14H, m), 1.55 (1H, dd, J=14, 6.6 Hz), 1.34 (1H, dd, J=14, 6.4 Hz), 0.92 (6H, d, J=6.4 Hz).
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-1,6-(p-phenylene)-2,3,4,5,20-pentanorprost-13-enoic Acid Methyl Ester
Less Polar
TLC: Rf 0.49 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.94 (2H, d, J=8.5 Hz), 7.25 (2H, d, J=8.5 Hz), 5.73 (1H, dt, J=15.5, 6.5 Hz), 5.43 (1H, dd, J=15.5, 8.5 Hz), 4.06 (1H, q, J=8.0 Hz), 3.90 (3H, s), 3.59 (1H, dd, J=9.5, 2.5 Hz), 3.00-2.60 (4H, m), 2.50-1.30 (14H, m), 0.93 (3H, t, J=7.5 Hz).
More Polar
TLC: Rf 0.43 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.94 (2H, d, J=8.0 Hz), 7.24 (2H, d, J=8.0 Hz), 5.69 (1H, ddd, J=15.0, 9.0, 5.5 Hz), 5.37 (1H, dd, J=15.0, 9.0 Hz), 4.03 (1H, q, J=8.5 Hz), 3.90 (3H, s), 3.57 (1H, dd, J=10.0, 2.0 Hz), 2.95-2.60 (3H, m), 2.50-1.30 (15H, m), 0.93 (3H, t, J=7.5 Hz).
To a suspension of the compound prepared in example 1 (10 mg, less polar) stirred in DMSO-phosphoric acid buffer (1 ml+1 ml) at room temperature, PLE (porcine liver esterase, 100 μl) was added, and the mixture was stirred at room temperature for 9 hours. The reaction was quenched by addition of a saturated aqueous solution of ammonium chloride, and the mixture was extracted by ethyl acetate. The organic layer was washed with brine/1N hydrochloric acid twice, with brine once, and dried over anhydrous magnesium sulfate, and concentrated. The residue was purified by column chromatography on silica gel (WAKO-C200 5 ml, ethyl acetate/hexane=1/1→1/0 to give the title compound (7 mg, less polar) having the following physical data as a colorless oil.
TLC: Rf 0.39 (ethyl acetate:hexane=3:1);
NMR (CDCl3): δ 7.98 (2H, d, J=8.0 Hz), 7.26 (2H, d, J=8.0 Hz), 5.75 (1H, dt, J=15.5, 6.5 Hz), 5.45 (1H, dd, J=15.5, 8.0 Hz), 4.08 (1H, q, J=9.0 Hz), 3.61 (1H, dd, J=10, 3.0 Hz), 3.00-2.60 (3H, m), 2.50-1.20 (19H, m), 0.95 (3H, t, J=7.0 Hz).
By the same procedure as described above, using the compound prepared in example 1 (more polar), the compound having the following physical data was obtained.
TLC: Rf 0.32 (hexane: ethyl acetate=1:3);
NMR (CDCl3): δ 7.99 (2H, d, J=8.2 Hz), 7.27 (2H, d, J=8.2 Hz), 5.71 (1H, ddd, J=15.4, 8.4, 5.2 Hz), 5.40 (1H, dd, J=15.4, 8.6 Hz), 4.15-3.95 (1H, m), 3.59 (1H, dd, J=10.0, 2.2 Hz), 3.00-1.50 (3H, br), 2.90-2.65 (3H, m), 2.50-1.20 (17H, m), 0.95 (3H, t, J=6.8 Hz).
By the same procedure as example 2, using the compounds prepared in example 1(a)-1(d), the compounds having the following physical data were obtained.
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13,19-dienoic Acid
More Polar
TLC: Rf 0.33 (hexane:ethyl acetate:acetic acid=1:2:0.03);
NMR (CDCl3): δ 7.99 (2H, d, J=8.2 Hz), 7.26 (2H, d, J=8.2 Hz), 5.95 (1H, ddt, J=17.0, 10.0, 7.4 Hz), 5.69 (1H, ddd, J=15.0, 8.6, 5.2 Hz), 5.38 (1H, dd, J=15.0, 9.0 Hz), 5.20-5.05 (2H, m), 5.00-3.00 (3H, br), 4.15-3.95 (1H, m), 3.59 (1H, dd, J=10.2, 2.2 Hz), 2.95-2.65 (3H, m), 2.50-1.65 (15H, m).
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-19,20-methano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid
More Polar
TLC: Rf 0.44 (hexane:ethyl:acetate=1:4);
NMR (CDCl3): δ 7.99 (2H, d, J=8.3 Hz), 7.25 (2H, d, J=8.3 Hz), 5.71 (1H, ddd, J=15, 9.1, 5.3 Hz), 5.38 (1H, dd, J=15, 8.7 Hz), 4.19 (3H, br), 4.05 (1H, m), 3.71 (1H, dd, J=9.4, 1.5 Hz), 2.78 (3H, m), 2.31 (3H, m), 2.17-1.61 (10H, m), 1.52 (1H, dd, J=14, 6.8 Hz), 1.38 (1H, dd, J=14, 6.4 Hz), 0.78 (1H, m), 0.51 (2H, m), 0.11 (2H, m).
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-19-methyl -1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid
More Polar
TLC: Rf 0.52 (hexane:ethyl acetate=1:4); NMR (CDCl3): δ 7.99 (2H, d, J=8.4 Hz), 7.25 (2H, d, J=8.4 Hz), 5.71 (1H, ddd, J=15, 9.4, 5.4 Hz), 5.40 (1H, dd, J=15, 9.0 Hz), 4.48 (3H, br), 4.06 (1H, m), 3.69 (1H, dd, J=9.0, 1.4 Hz), 2.78 (3H, m), 2.50-1.62 (14H, m), 1.56 (1H, dd, J=14, 6.7 Hz), 1.35 (1H, dd, J=14, 6.3 Hz), 0.92 (6H, d, J=6.2 Hz).
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-1,6-(p-phenylene)-2,3,4,5,20-pentanorprost-13-enoic Acid
More Polar
TLC: Rf 0.39 (ethyl acetate:hexane:acetic acid 9:3:0.1);
NMR (CDCl3): δ 7.99 (2H, d, J=8.0 Hz), 7.26 (2H, d, J=8.0 Hz), 5.70 (1H, ddd, J=14.0, 8.5, 5.0 Hz), 5.39 (1H, dd, J=14.0, 9.0 Hz), 4.05 (1H, q, J=8.0 Hz), 3.60 (1H, dd, J=10, 2.0 Hz), 2.90-2.65 (3H, m), 2.50-2.15 (3H, m), 2.15-1.30 (14H, m), 0.93 (3H, t, J=7.5 Hz).
To a solution of 4-(t-butyldimethylsilyloxy) cyclopent-2-en-1-one (466 mg) in methanol (5 ml), was added dropwise an aqueous solution of hydrogen peroxide (1.00 ml, 31%) under cooling with ice, and the mixture was stirred for 15 minutes. To the reaction solution was added a saturated aqueous solution of ammonium chloride. The mixture was extracted with ethyl acetate twice, and the combined organic layer was washed with a saturated aqueous solution of sodium thiosulfate, with a saturated aqueous solution of sodium chloride, successively. The solution was dried over magnesium sulfate and concentrated. To a solution of the crude product in chloroform (15 ml), alumina (5 g) and 4-mercaptomethyl methyl benzoate (3.43 g) were added and the mixture was stirred for 15 hours. The reaction solution was filtered, and the filtrate was concentrated in vacuo to give a crude product. The crude product was purified by column chromatography on silica gel (ethyl acetate - hexane) to give the title compound (611 mg) having the following physical data.
TLC: Rf 0.38 (hexane:ethyl acetate=1:1);
NMR (CDCl3): δ 8.00 (2H, d, J=8.0 Hz), 7.42 (2H, d, J=8.0 Hz), 6.83 (1H, d, J=3.0 Hz), 4.87 (1H, m), 4.15 (1H, d, J=13.0 Hz), 4.07 (1H, d, J=13.0 Hz), 3.90 (3H, s), 2.81 (1H, dd, J=19.0, 6.0 Hz), 2.33 (1H, dd, J=19.0, 2.0 Hz), 0.90 (9H, s), 0.06 (6H, s).
(11α,8Z, 13E)-9-Acetyloxy-11,16-bis(t-butyldimethylsilyloxy)-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5,20-pentanorprost-8,13-dienoic Acid Methyl Ester
Under atmosphere of argon, to a solution of 1-iodo-4-t-butyldimethylsilyloxy-5,5-propano-1-heptene (400 mg) in diethyl ether (10 ml) stirred at -78° C., t-butyllithium/pentane solution (1.24 ml, 1.64 M) was added dropwise slowly, and the mixture was stirred at −78° C. for 45 minutes. Lithium cyano thiophenyl cuprate/THF solution (4.8 ml, 0.25 M) was added therein, and the mixture was stirred for 20 minutes. To the mixture, a solution of the compoundprepared in reference example 5 (362 mg) in diethyl ether (6 ml) was added dropwise slowly, and warmed from −78° C. to −10° C. over a period of 1.5 hours. To the reaction solution, acetic anhydride (0.13 ml) was added dropwise, and the mixture was stirred at a temperature from −10° C. to 0° C. for 15 minutes. The reaction was quenched by addition of a saturated aqueous solution of ammonium chloride, and the mixture was extracted with ethyl acetate three times. The organic layer was washed with a saturated aqueous solution of ammonium chloride twice, with brine once, dried over anhydrous magnesium sulfate, and concentrated. The residue was purified by column chromatography on silica gel (Merck Kiesel gel 7734, 50 ml, ethyl acetate/hexane=1/15) to give the title compound (422 mg) having the following physical data as a light yellow oil.
TLC: Rf 0.31 (ethyl acetate:hexane=1:10);
NMR (CDCl3): δ 7.93 (2H, d, J=8.5 Hz), 7.32 (2H, d, J=8.5 Hz), 5.70-5.50 (1H, m), 5.30-5.10 (1H, m), 4.10-4.00 (1H, m), 3.89 (3H, s), 3.85-3.78 (2H, m), 3.57 (1H, t, J=5.0 Hz), 3.10-3.00 (1H, m), 2.95-2.80 (1H, m), 2.42-2.30 (1H, m), 2.20-2.10 (2H, m), 2.07 (3H, s), 2.00-1.30 (5H, m), 1.00-0.90 (3H, s), 0.89 (9H, s), 0.84 (9H, s), 0.10-0.00 (6H, m), 0.00 (6H, s).
(11α,8Z, 13E)-9-Acetyloxy-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5,20-pentanorprost-8,13-dienoic acid Methyl Ester
By the same procedure as example 1, using the compound prepared in reference example 6, the compounds having the following physical data were obtained.
Less Polar
TLC: Rf 0.59 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.98 (2H, d, J=8.5 Hz), 7.37 (2H, d, J=8.5 Hz), 5.55 (1H, ddd, J=15.0, 8.5, 6.0 Hz), 5.26 (1H, dd, J=15.0, 9.0 Hz), 4.20-4.05 (1H, m), 3.91 (3H, s), 3.86 (2H, s), 3.49 (1H, dd, J=10.0, 2.5 Hz), 3.10-3.00 (1H, m), 2.92 (1H, ddd, J=16.5, 7.0, 1.0 Hz), 2.49 (1H, ddd, J=16.5, 12.5, 1.0 Hz), 2.30-1.30 (12H, m), 2.15 (3H, s), 0.92 (3H, t, J=7.5 Hz).
More Polar
TLC: Rf 0.52 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.97 (2H, d, J=8.0 Hz), 7.37 (2H, d, J=8.0 Hz), 5.61 (1H, ddd, J=15.0, 8.5, 7.0 Hz), 5.35 (1H, dd, J=15.0, 8.5 Hz), 4.20-4.05 (1H, m), 3.91 (3H, s), 3.86 (2H, s),3.54 (1H, dd, J=9.0, 3.0 Hz), 3.08 (1H, dd, J=8.5, 3.0 Hz), 2.92 (1H, ddd, J=16.0, 6.5, 1.5 Hz), 2.46 (1H, ddd, J=16.0, 4.0, 1.5 Hz), 2.30-2.10 (1H, m), 2.15 (3H, s), 2.10-1.30 (10H, m), 0.92 (3H, t, J=7.5 Hz).
By the same procedure as reference example 1, 2, 3, 4 and example 3, the compounds having the following physical data were obtained.
(11α,8Z, 13E)-9-Acetyloxy-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-8,13-dienoic Acid Methyl Ester
Less Polar
TLC Rf 0.55 (ethyl acetate:hexane=3 2);
NMR (CDCl3): δ 7.97 (2H, d, J=8.0 Hz), 7.37 (2H, d, J=8.0 Hz), 5.55 (1H, ddd, J=15.0, 8.5, 5.5 Hz), 5.26 (1H, dd, J=15.0, 9.0 Hz), 4.20-4.05 (1H, m), 3.91 (3H, s), 3.86 (2H, s), 3.49 (1H, dd, J=10.0, 2.0 Hz), 3.07 (1H, dd, J=9.0, 4.0 Hz), 2.92 (1H, ddd, J=16.5, 7.0, 2.0 Hz), 2.49 (1H, ddd, J=16.5, 4.0, 2.0 Hz), 2.40-1.20 (15H, m), 2.15 (3H, s), 0.94 (3H, t, J=6.5 Hz).
More Polar
TLC: Rf 0.50 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.97 (2H, d, J=8.0 Hz), 7.36 (2H, d, J=8.0 Hz), 5.56 (1H, ddd, J=15.5, 8.0, 5.5 Hz), 5.36 (1H, dd, J=15.5, 8.5 Hz), 4.20-4.05 (1H, m), 3.90 (3H, s), 3.88 (2H, s), 3.52 (1H, dd, J=10.0, 2.5 Hz), 3.09 (1H, dd, J=8.5, 3.5 Hz), 2.90 (1H, ddd, J=16.5, 7.0, 2.0 Hz), 3.00-2.60(1H, brs), 2.46 (1H, ddd, J=16.0, 4.0, 1.5 Hz), 2.25-2.15 (1H, m), 2.15 (3H, s), 2.10-1.20 (13H, m), 0.94 (3H, t, J=6.5 Hz).
(11α,8Z, 13E)-9-Acetyloxy-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-8,13,19-trienoic Acid Methyl Ester
Less Polar
TLC Rf 0.59 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.98 (2H, d, J=8.5 Hz), 7.38 (2H, d, J=8.5 Hz), 6.06-5.82 (1H, m), 5.54 (1H, ddd, J=15.5, 8.5, 6.0 Hz), 5.26 (1H, dd, J=15.5, 8.5 Hz), 5.18-5.04 (2H, m), 4.20-4.05 (1H, m), 3.91 (3H, s), 3.86 (2H, s), 3.50 (1H, dd, J=10.0, 2.5 Hz), 3.10-3.00 (1H, m), 2.92 (1H, ddd, J=16.5, 7.0, 1.5 Hz), 2.60-1.30 (13H, m), 2.15 (3H, s).
More Polar
TLC: Rf 0.52 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.97 (2H, d, J=8.5 Hz), 7.36 (2H, d, J=8.5 Hz), 6.06-5.82 (1H, m), 5.62 (1H, ddd, J=15.5, 8.5, 5.5 Hz), 5.35 (1H, dd, J=15.0, 8.5 Hz), 5.20-4.94 (2H, m), 4.20-4.05 (1H, m), 3.90 (3H, s), 3.88 (2H, s), 3.53 (1H, dd, J=10.0, 2.5 Hz), 3.08 (1H, dd, J=6.0, 1.5 Hz), 2.91 (1H, ddd, J=16.5, 7.0, 2.0 Hz), 2.55-1.30 (13H, m), 1.26 (3H, t, J=7.0 Hz).
(11α,8z, 13E)-9-Acetyloxy-11,16-dihydroxy-19-methyl -17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-8,13-dienoic Acid Methyl Ester
Less Polar
TLC Rf 0.45 (ethyl acetate hexane=1:1);
NMR (CDCl3): δ 7.98 (2H, d, J=8.5 Hz), 7.38 (2H, d, J=8.5 Hz), 5.56 (1H, ddd, J=14.5, 8.5, 6.0 Hz), 5.27 (1H, dd, J=14.5, 9.0 Hz), 4.20-4.05 (1H, m), 3.91 (3H, s), 3.88 (2H, s), 3.58 (1H, dd, J=10.0, 2.0 Hz), 3.14-3.00 (1H, m), 2.92 (1H, ddd, J=16.0, 6.0, 1.5 Hz), 2.49 (1H, ddd, J=16.0, 4.0, 1.5 Hz), 2.35-2.20 (1H, m), 2.20-1.50 (10H, m), 2.15 (3H, s), 1.33 (1H, dd, J=14.0, 6.0 Hz), 0.93 (3H, d, J=6.5 Hz), 0.93 (3H, d, J=6.5 Hz).
More Polar
TLC: Rf 0.38 (ethyl acetate:hexane=1:1);
NMR (CDCl3): δ 7.97 (2H, d, J=8.0 Hz), 7.37 (2H, d, J=8.0 Hz), 5.75-5.55 (1H, m), 5.38 (1H, dd, J=15.5, 8.0 Hz), 4.20-4.05 (1H, m), 3.91 (3H, s), 3.88 (2H, s), 3.62 (1H, d, J=10.0 Hz), 3.09 (1H, d, J=8.5 Hz), 2.93 (1H, ddd, J=16.5, 6.5, 1.5 Hz), 2.46 (1H, dd, J=16.5, 3.5 Hz), 2.40-2.20 (2H, m), 2.16 (3H, s), 2.15-1.50 (9H, m), 1.55 (1H, dd, J=14.5, 7.0 Hz), 1.33 (1H, dd, J=14.5, 6.5 Hz), 0.91 (6H, d, J=6.5 Hz).
(11α,8Z, 13E)-9-Acetyloxy-11,16-dihydroxy-17,17-propano-19,20-methano-7-thia-1,6-(p-phenylene)-2,3,4,5tetranorprost-8,13-dienoic Acid Methyl Ester
Less Polar
TLC Rf 0.38 (ethyl acetate:hexane=1:1);
NMR (CDCl3): δ 7.97 (2H, d, J=8.0 Hz), 7.37 (2H, d, J=8.0 Hz), 5.57 (1H, ddd, J=15.0, 8.5, 6.0 Hz), 5.26 (1H, dd, J=15.0, 9.0 Hz), 4.20-4.15 (1H, m), 3.90 (3H, s), 3.86 (2H, s), 3.64 (1H, dd, J=10.0, 2.0 Hz), 3.06 (1H, dd, J=8.5, 3.0 Hz), 2.92 (1H, ddd, J=16.5, 7.0, 1.5 Hz), 2.49 (1H, ddd, J=16.5, 4.0, 1.5 Hz), 2.35-2.20 (1H, m), 2.15 (3H, s), 2.10-1.50 (8H, m), 1.55 (1H, dd, J=14.0, 6.5 Hz), 1.33 (1H, dd, J=14.0, 6.0 Hz), 0.90-0.70 (1H, m), 0.55-0.45 (1H, m), 0.15-0.05 (1H, m).
More Polar
TLC: Rf 0.32 (ethyl acetate: hexane=1:1);
NMR (CDCl3): δ 7.97 (2H, d, J=8.0 Hz), 7.36 (2H, d, J=8.0 Hz), 5.70-5.50 (1H, m), 5.35 (1H, dd, J=15.5, 8.5 Hz), 4.20-4.05 (1H, 5 m), 3.91 (3H, s), 3.88 (2H, s), 3.67 (1H, dd, J=9.5, 2.0 Hz), 3.15-3.05 (1H, m), 2.92 (1H, dd, J=16.0, 6.5 Hz), 2.55-1.60 (7H, m), 1.51 (1H, dd, J=14.5, 7.0 Hz), 1.35 (1H, dd, J=14.5, 6.0 Hz), 0.90-0.70 (1H, m), 0.60-0.45 (2H, m), 0.15-0.05 (2H, m).
(11α,13E) -9-Oxo-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5,20-pentanorprost-13-enoic Acid Methyl Ester
To a solution of the compound prepared in example 3 (112 mg, more polar) in DMSO/phosphoric acid buffer (3 ml+3 ml) stirred at room temperature, was added lipase (200 mg, Amano-PS), and the mixture was stirred at room temperature for 2 hours. The reaction was quenched by addition of a saturated solution of sodium sulfate. The mixture was extracted with ethyl acetate three times. The organic layer was washed with water twice, with brine once. The extract was dried over anhydrous magnesium sulfate and concentrated. The residue was purified by column chromatography on silica gel (Merck Kiesel gel 7734, 20 ml, ethyl acetate/hexane=1/1→2/1) to give the title compound (77 mg) having the following physical data as a light yellow oil.
More Polar
TLC: Rf 0.36, 0.40 (ethyl acetate:hexane=3:2); NMR (CDCl3): δ 7.97 (2H, d, J=8.0 Hz), 7.44, 7.42 (2H, each-d, J=8.0 Hz), 5.80-5.16 (2H, m), 4.50-3.70 (4H, m), 3.91 (3H, s), 3.52 (1H, dd, J=10.0, 2.0 Hz), 3.24-2.64 (3H, m), 2.50-1.20 (12H, m), 0.91 (3H, t, J=7.0 Hz).
By the same procedure as described above, using the compound prepared in example 3 (less polar), the compound having the following physical data was obtained.
Less Polar
TLC: Rf 0.40 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.98 (2H, d, J=8.0 Hz), 7.44, 7.43 (2H, each-d, J=8.0 Hz), 5.75-5.22 (2H, m), 4.52-3.70 (3H, m), 3.91 (3H, s), 3.51 (1H, dd, J=9.0, 2.5 Hz), 3.30-2.68 (3H, m), 2.50-2.10 (3H, m), 2.10-1.30 (10H, m), 0.92 (3H, t, J=7.5 Hz).
By the same procedure as example 4, using the compounds prepared in example 3(a)-3(d), the compounds having the following physical data were obtained.
(11α,13E) -9-Oxo-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
Less Polar
TLC: Rf 0.49 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.98 (2H, d, J=8.0 Hz), 7.42 (2H, d, J=8.0 Hz), 5.75-5.23 (2H, m), 4.50-3.70 (3H, m), 3.91 (3H, s), 3.50 (1H, dd, J=10.0, 2.0 Hz), 3.30-2.55 (3H, m), 2.55-2.10 (3H, m), 2.10-1.20 (12H, m), 0.94 (3H, t, J=6.0 Hz). more polar
TLC: Rf 0.42, 0.49 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.97 (2H, d, J=8.0 Hz), 7.43, 7.42 (2H, each-d, J=8.0 Hz), 5.75-5.18 (2H, m), 4.50-3.62 (3H, m), 3.90 (3H, s), 3.52 (1H, dd, J=10.0, 2.0 Hz), 3.22-2.65 (3H, m), 2.55-1.20 (15H, m), 0.94 (3H, t, J=6.5 Hz).
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13,19-dienoic Acid Methyl Ester
Less Polar
TLC: Rf 0.40 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.98 (2H, d, J=8.0 Hz), 7.43, 7.42 (2H, each-d, J=8.0 Hz), 6.16-5.80 (1H, m), 5.75-5.22 (2H, m), 5.18-5.02 (2H, m), 4.50-3.70 (3H, m), 3.91 (3H, s), 3.51 (1H, dd, J=9.5, 2.0 Hz), 3.30-2.67 (2H, m), 2.60-1.50 (13H, m).
More Polar
TLC: Rf 0.36, 0.40 (ethyl acetate:hexane=3:2); NMR (CDCl3): δ 7.97 (2H, d, J=8.0 Hz), 7.43, 7.41 (2H, each-d, J=8.0 Hz), 6.50-5.80 (1H, m), 5.70-5.20 (2H, m), 5.20-5.00 (2H, m), 4.50-3.68 (4H, m), 3.91 (3H, s), 3.51 (1H, d, J=10.0 Hz), 3.22-2.65 (2H, m), 2.50-1.60 (12H, m).
(11α,13E)-9-Oxo-11,16-dihydroxy-19-methyl-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
Less Polar
TLC Rt 0.60 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.99 (2H, d, J=8.0 Hz), 7.44, 7.43 (2H, each-d, J=8.0 Hz), 5.80-5.25 (2H, m), 4.55-3.70 (3H, m), 3.91 (3H, s), 3.64-3.52 (1H, m), 3.30-2.70 (2H, m), 2.60-1.40 (14H, m), 1.40-1.20 (1H, m), 0.93, 0.92 (6H, each-d, J=6.5 Hz).
More Polar
TLC: Rf 0.52, 0.58 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.98 (2H, d, J=8.0 Hz), 7.44, 7.43 (2H, each-d, J=8.0 Hz), 5.80-5.20 (2H, m), 4.55-3.70 (3H, m), 3.91 (3H, s), 3.62 (1H, d, J=10.0 Hz), 3.24-2.68 (3H, m), 2.55-2.20 (3H, m), 2.20-1.60 (10H, m), 1.54 (1H, dd, J=14.0, 6.5 Hz), 1.32 (1H, dd, J=14.0, 5.5 Hz), 0.91, 0.90 (6H, each-d, J=6.5 Hz).
(11α,13E) -9-Oxo-11,16-dihydroxy-17,17-propano-19,20-methano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
Less Polar
TLC Rf 0.54 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.98 (2H, d, J=8.0 Hz), 7.44, 7.43 (2H, each-d, J=8.0 Hz), 5.80-5.24 (2H, m), 4.55-3.70 (3H, m), 3.91 (3H, s), 3.70-3.60 (1H, m), 3.30-2.70 (2H, m), 2.60-2.15 (4H, m), 2.10-1.60 (8H, m), 1.52 (1H, dd, J=14.0, 6.5 Hz), 1.34 (1H, dd, J=14.0, 6.0 Hz), 1.00-0.65 (1H, m), 0.60-0.45 (2H, m), 0.16-0.05 (2H, m).
More Polar
TLC: Rf 0.43, 0.49 (ethyl acetate:hexane=3:2);
NMR (CDCl3): δ 7.98 (2H, d, J=8.0 Hz), 7.44, 7.42 (2H, each-d, J=8.0 Hz), 5.80-5.16 (2H, m), 4.50-3.76 (3H, m), 3.91 (3H, s), 3.66 (1H, dd, J=10.5, 2.0 Hz), 3.22-2.66 (3H, m), 2.55-2.20 (3H, m), 2.10-1.60 (8H, m), 1.60-1.30 (2H, m), 0.85-0.68 (1H, m), 0.58-0.45 (2H, m), 0.18-0.04 (2H, m).
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5,20-pentanorprost-13-enoic Acid
By the same procedure as example 2, using the compound prepared in example 4 (more polar), the compound having the following physical data was obtained.
More Polar
TLC: Rf 0.32, 0.37 (ethyl acetate:hexane:acetic acid 6:4:0.1);
NMR (CDCl3): δ 8.03 (2H, d, J=8.5 Hz), 7.45 (2H, d, J=8.5 Hz), 5.70-5.20 (2H, m), 4.54-3.70 (3H, m), 3.56 (1H, dd, J=9.5, 2.0 Hz), 3.25-1.30 (16H, m), 0.92 (3H, t, J=7.5 Hz).
By the same procedure as example 2, using the compounds prepared in example 4(a)-4(d), the compounds having the following physical data were obtained.
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid
Less Polar
TLC Rf 0.34 (ethyl acetate:hexane:acetic acid=200:100:1);
NMR (CDCl3): δ 8.02 (2H, d, J=8.0 Hz), 7.45 (2H, d, J=8.0 Hz), 5.80-5.25 (2H, m), 4.55-3.66 (4H, m), 4.25 (3H, brs), 3.53 (1H, dd, J=10.0, 2.5 Hz), 3.30-2.10 (5H, m), 2.10-1.20 (10H, m), 0.93 (3H, t, J=7.0 Hz).
More Polar
TLC: Rf 0.29, 0.34 (ethyl acetate:hexane:acetic acid=200 100:1);
NMR (CDCl3): δ 8.02 (2H, d, J=8.0 Hz), 7.44 (2H, d, J=8.0 Hz), 5.80-5.20 (2H, m), 4.55-3.70 (4H, m), 3.55 (1H, dd, J=10.0, 2.0 Hz), 3.40-2.65 (2H, m), 3.20 (3H, brs), 2.60-2.20 (3H, m), 2.10-1.20 (10H, m), 0.94 (3H, t, J=6.5 Hz).
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13,19-dienoic Acid
More Polar
TLC: Rf 0.33, 0.39 (ethyl acetate:hexane:acetic acid=6:4:0.1);
NMR (CDCl3): δ 8.03 (2H, d, J=8.0 Hz), 7.46 (2H, d, J=8.0 Hz), 6.05-5.80 (1H, m), 5.70-5.50 (1H, m), 5.36-5.20 (1H, m), 5.20-5.04 (2H, m), 4.20-3.70 (3H, m), 3.54 (1H, dd, J=10.0, 3.0 Hz), 2.85-2.60 (2H, m), 2.60-1.60 (14H, m).
(11α,13E)-9-Oxo-11,16-dihydroxy-19-methyl-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid
More Polar
TLC: Rf 0.32, 0.37 (ethyl acetate hexane:acetic acid=6:3:0.1);
NMR (CDCl3): δ 8.02 (2H, d, J=8.0 Hz), 7.46, 7.45 (2H, each-d, J=8.0 Hz), 5.80-5.20 (2H, m), 4.52-3.95 (2H, m), 3.95-3.60 (2H, m), 3.30-2.70 (3H, m), 2.65-2.20 (3H, m), 2.15-1.60 (9H, m), 1.54 (1H, dd, J=14.0, 7.0 Hz), 1.33 (1H, dd, J=14, 6.5 Hz), 0.91 (6H, d, J=6.5 Hz).
(11α,13E) -9-Oxo-11,16-dihydroxy-17,17-propano-19,20-methano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid
More Polar
TLC: Rf 0.25, 0.31 (ethyl acetate:hexane:acetic acid=6:3:0.1);
NMR (CDCl3): δ 8.02 (2H, d, J=8.0 Hz), 7.46, 7.45 (2H, each-d, J=8.0 Hz), 5.70-5.16 (2H, m), 4.52-3.62 (5H, m), 3.30-2.70 (3H, m), 2.65-2.20 (3H, m), 2.10-1.60 (7H, m), 1.50 (1H, dd, J=14.0, 6.5 Hz), 1.38 (1H, dd, J=14, 6.0 Hz), 0.85-0.70 (1H, m), 0.58-0.45 (2H, m), 0.16-0.05 (2H, m).
(11α,13E) -9-Oxo-11,16-bis (t-butyldimethylsilyloxy) -17,17-propano-6-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
Under atmosphere of argon, to a solution of the compound prepared in reference example 3 (102 mg) in methanol (2 ml), were added 4-mercapto methyl benzoate (32 mg) and piperidine (10 μl) at −78° C. The mixture was stirred at −78° C. for 1 hour. To this solution, a saturated aqueous solution of ammonium chloride was added. The mixture was extracted with ethyl acetate. The organic layer was washed, dried, and then concentrated. The residue was purified by column chromatography on silica gel to give the title compound (77 mg) having the following physical data.
TLC: Rf 0.58 (hexane:ethyl acetate=4:1).
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-6-thia-1,6(p-phenylene)-2,3,4,5-tetranorprost-13-enoic acid methyl ester
To a solution of the compound prepared in reference example 7 (76 mg) in acetonitrile (20 ml), was added an aqueous solution of hydrofluoric acid (1 ml; 47%) under cooling with ice, and the mixture was stirred at room temperature for 1.5 hours. To this solution, was added a saturated aqueous solution of sodium bicarbonate. The mixture was vigorously stirred, and extracted with ethyl acetate. The organic layer was washed with a saturated aqueous solution of sodium bicarbonate, with brine, and dried, and then concentrated in vacuo. The residue was purified by Lobar column (Lobar column size A, hexane/ethyl acetate=2/3) to give the title compounds (less polar: 18 mg, more polar: 16 mg) having the following physical data.
Less Polar
TLC: Rf 0.42 (hexane:ethyl acetate=2:3);
NMR (CDCl3): δ 7.94 (2H, d, J=8.4 Hz), 7.35 (2H, d, J=8.4 Hz), 5.64 (1H, ddd, J=15.0, 8.2, 5.6 Hz), 5.47 (1H, dd, J=15.0, 8.2 Hz), 4.20-4.05 (1H, m), 3.90 (3H, s), 3.54 (1H, dd, J=10.0, 2.2 Hz), 3.43 (1H, dd, J=13.3, 4.1 Hz), 3.14 (1H, dd, J=13.3, 6.7 Hz), 2.81 (1H, ddd, J=18.6, 7.8, 1.0 Hz), 2.61 (1H, dt, J=11.6, 8.2 Hz), 2.45-2.20 (2H, m), 2.29 (1H, dd, J=18.6, 9.2 Hz), 2.10-1.20 (13H, m), 0.95 (3H, t, J=6.7 Hz).
More Polar
TLC: Rf 0.34 (hexane:ethyl acetate=2:3);
NMR (CDCl3): δ 7.93 (2H, d, J=8.6 Hz), 7.33 (2H, d, J=8.6 Hz), 5.62 (1H, ddd, J=15.2, 7.8, 5.4 Hz), 5.42 (1H, dd, J=15.2, 8.2 Hz), 4.15-4.00 (1H, m), 3.90 (3H, s), 3.54 (1H, dd, J=9.8, 2.6 Hz), 3.35 (1H, dd, J=13.4, 4.6 Hz), 3.19 (1H, dd, J=13.4, 5.7 Hz), 2.79 (1H, ddd, J=18.8, 7.6, 1.0 Hz), 2.60 (1H, dt, J=12.2, 8.2 Hz), 2.45-2.20 (2H, m), 2.28 (1H, dd, J=18.8, 9.4 Hz), 2.10-1.20 (13H, m), 0.95 (3H, t, J=6.8 Hz).
(11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-6-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid
By the same procedure as example 2, using the compounds prepared in example 6, the title compounds having the following physical data were obtained.
Less Polar
TLC: Rf 0.33 (hexane:ethyl acetate:acetic acid=2:3:0.05);
NMR (CDCl3): δ 7.96 (2H, d, J=8.4 Hz), 7.35 (2H, d, J=8.4 Hz), 5.66 (1H, ddd, J=15.4, 7.8, 5.4 Hz), 5.49 (1H, dd, J=15.4, 7.8 Hz), 4.20-4.05 (1H, m), 4.00-2.50 (3H, br), 3.57 (1H, dd, J=10.0, 2.2 Hz), 3.43 (1H, dd, J=13.4, 4.0 Hz), 3.16 (1H, dd, J=13.4, 6.4 Hz), 2.82 (1H, ddd, J=18.8, 7.2, 1.0 Hz), 2.62 (1H, dt, J=12.0, 8.4 Hz), 2.50-2.20 (2H, m), 2.30 (1H, dd, J=18.8, 9.2 Hz), 2.15-1.20 (13H, m), 0.94 (3H, t, J=6.7 Hz).
More Polar
TLC: Rf 0.27 (hexane ethyl acetate:acetic acid=2:3:0.05);
NMR (CDCl3): δ 7.96 (2H, d, J=8.4 Hz), 7.34 (2H, d, J=8.4 Hz), 5.70-5.35 (2H, m), 4.20-4.00 (1H, m), 4.00-2.50 (3H, br), 3.58 (1H, d, J=10.0 Hz), 3.35 (1H, dd, J=13.8, 4.4 Hz), 3.20 (1H, dd, J=13.8, 5.6 Hz), 2.80 (1H, dd, J=18.8, 7.2 Hz), 2.73-2.55 (1H, m), 2.50-2.15 (3H, m), 2.15-1.20 (11H, m), 0.94 (3H, t, J=6.9 Hz).
(9α,11α,13E)-9-Hydroxy-11,16-bis(t-butyldimethylsilyloxy)-17,17-propano-19,20-methano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
Under atmosphere of argon, to a solution of (11α,13E)-9-oxo-11,16-bis(t-butyldimethylsilyloxy)-17,17-propano-19,20-methano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic acid methyl ester (170 mg, an intermediate in example 1(b); which was prepared by the same procedure as provided in reference example 1,2,3 and 4) in anhydrous THF (2.5 ml), was added lithium tri-s-butyl borohydride (1.0 M, 279 μl) was added dropwise at −78° C., and the mixture was stirred at −78° C. for 1.5 hours. To the mixture, was added dropwise an aqueous solution of hydrogen peroxide (0.5 ml), and the mixture was warmed to 0° C. The mixture was acidified by addition of 1N hydrochloric acid, and the mixture was extracted with ethyl acetate. The organic layer was washed, dried, concentrated in vacuo. The residue was purified by column chromatography on silica gel (Merck 7734, 10 g, hexane/ethyl acetate=25/1→15/1) to give the title compound (102 mg) having the following physical data.
TLC: Rf 0.36 (hexane:ethyl acetate=9:1);
NMR (CDCl3): δ 7.94 (2H, d, J=8.1 Hz), 7.26 (2H, d, J=8.1 Hz), 5.41 (1H, m), 5.17 (1H, m), 4.17 (1H, m), 4.04 (1H, m), 3.90 (3H, s), 3.64 (1H, t, J=5.1 Hz), 3.00-2.50 (3H, m), 2.31-1.41 (14H, m), 1.22 (2H, m), 0.86 (19H, m), 0.47 (2H, m), 0.05 (14H, m).
(9α,11α,13E)-9-Acetyloxy-11,16-dihydroxy-17,17-propano-19,20-methano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
To a solution of the compound prepared in reference example 8 (80 mg) and dimethylaminopyridine (5 mg) in pyridine (2 ml), was added dropwise acetic anhydride (112 ml), and the mixture was stirred at room temperature for 2.5 hours. The reaction mixture was poured into ice-water. The mixture was extracted with ethyl ether. The organic layer was washed, dried, concentrated in vacuo, to give a crude product. To a solution of the crude product in acetonitrile (7 ml), was added dropwise an aqueous solution of hydrofluoric acid (0.35 ml, 47%), and the mixture was stirred at room temperature for 1.5 hours. The reaction mixture was poured into cooled ethyl acetate/a saturated aqueous solution of sodium bicarbonate. The mixture was extracted with ethyl acetate. The organic layer was washed, dried, and concentrated in vacuo. The residue was purified by column chromatography on silica gel (Lobar size A, hexane/ethyl acetate=3/2→1/1) to give the title compounds (less polar: 25 mg, more polar: 24 mg) having the following physical data. less polar
TLC: Rf 0.46 (hexane:ethyl acetate=2:3);
NMR (CDCl3): δ 7.94 (2H, d, J=8.3 Hz), 7.20 (2H, d, J=8.3 Hz), 5.66 (1H, m), 5.32 (1H, dd, J=14, 8.8 Hz), 5.22 (1H, m), 3.90 (3H, s), 3.87 (1H, m), 3.68 (1H, dd, J=9.8, 1.9 Hz), 2.79-1.43 (17H, m), 2.09 (3H, s), 1.34 (1H, dd, J=14, 6.2 Hz), 0.78 (1H, m), 0.49 (2H, m), 0.10 (2H, m).
More Polar
TLC: Rf 0.39 (hexane:ethyl acetate=2:3);
NMR (CDCl3): δ 7.94 (2H, d, J=8.4 Hz), 7.20 (2H, d, J=8.4 Hz), 5.61 (1H, ddd, J=15, 8.9, 5.9 Hz), 5.25 (2H, m), 3.90 (3H, s), 3.82 (1H, m), 3.66 (1H, dd, J=10, 2.4 Hz), 2.80-1.42 (17H, m), 2.09 (3H, s), 1.34 (1H, dd, J=14, 5.9 Hz), 0.77 (1H, m), 0.49 (2H, m), 0.10 (2H, m).
(9α,11α,13E)-9-Hydroxy-11,16-bis(t-butyldimethylsilyloxy) -17,17-propano-19,20-methano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
Under atmosphere of argon, to a solution of the compound prepared in reference example 9 (22 mg, more polar) in anhydrous methylene chloride (0.5 ml) were added dropwise, 2,6-lutidine (16 μl) and trifluoromethanesulfonic acid t-butyldimethylsilyl ester (25 μl), and the mixture was stirred at room temperature for 2 hours. To the reaction mixture, water was added. The mixture was extracted with ethyl acetate. The organic layer was washed, dried, and concentrated in vacuo to give a crude compound.
To a solution of the crude compound in anhydrous methanol (1 ml), potassium carbonate (8 mg) was added, and the mixture was stirred for 15 hours. The mixture was acidified by addition of 1N hydrochloric acid at 0° C., and the mixture was extracted with ethyl acetate. The organic layer was washed, dried, and concentrated in vacuo. The residue was purified by column chromatography on silica gel (Merck 7734, 2 g, hexane/ethyl acetate=25/1→15/1) to give the title compound (28 mg) having the following physical data.
TLC: Rf 0.33 (hexane:ethyl acetate=9:1);
NMR (CDCl3): 7.94 (2H, d, J=8.2 Hz), 7.26 (2H, d, J=8.2 Hz), 5.45 (1H, dt, J=15, 7.4 Hz), 5.17 (1H, dd, J=15, 8.6 Hz), 4.17 (1H, m), 3.99 (1H, m), 3.89 (3H, s), 3.64 (1H, t, J=7.3 Hz), 2.96-2.52 (3H, m), 2.29-1.41 (14H, m), 1.21 (1H, dd, J=14, 6.7 Hz), 0.88 (19H, m), 0.47 (2H, m), 0.05 (14H, m).
(9α,11α,13E) -9-Tosyloxy-11,16-bis (t-butyldimethylsilyloxy) -17,17-propano-19,20-methano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
Under atmosphere of argon, to a solution of the compound prepared in reference example 10 (28 mg) in anhydrous pyridine (1.5 ml), was added tosyl chloride (80 mg) at 0° C. The mixture was stirred at room temperature overnight. To the reaction solution was added water (0.5 ml), and the mixture was stirred for 5 minutes. The mixture was extracted with ethyl acetate. The organic layer was washed, dried, concentrated in vacuo to give the title compound (34 mg) having the following physical data.
TLC: Rf 0.82 (toluene:isopropylalcohol=30:1).
(9β,11α,13E) -9-Chloro-11,16-bis(t-butyldimethylsilyloxy) -17,17-propano-19,20-methano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
Under atmosphere of argon, to a solution of the compound prepared in reference example 11 (34 mg) in anhydrous toluene (2 ml), tetrabutylammonium chloride (116 mg) was added quickly, and the mixture was stirred at 55° C. for 1 hour. The reaction mixture was diluted with ethyl acetate, washed, dried and concentrated in vacuo to give the title compound having the following physical data.
TLC: Rf 0.88 (toluene:isopropylalcohol=30:1).
(9β,11α,13E)-9-Chloro-11,16-dihydroxy-17,17-propano -19,20-methano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
By the same procedure as example 1, using the compound prepared in reference example 12, the title compound having the following physical data was obtained.
More Polar
TLC: Rf 0.58 (hexane:ethyl acetate=1:1);
NMR (CDCl3): δ 7.94 (2H, d, J=8.3 Hz), 7.26 (2H, d, J=8.3 Hz), 5.59 (1H, ddd, J=15, 8.6, 5.4 Hz), 5.38 (1H, dd, J=15, 8.0 Hz), 4.09 (2H, m), 3.90 (3H, s), 3.66 (1H, dd, J=10, 2.0 Hz), 3.08 (1H, bs), 2.78 (2H, t, J=7.8 Hz), 2.40-1.62 (14H, m), 1.50 (1H, dd, J=14, 6.6 Hz), 1.36 (1H, dd, J=14, 6.3 Hz), 0.77 (1H, m), 0.50 (2H, m), 0.10 (2H, m).
By the same procedure as reference example 8, 9, 10, 11, 12 and example 1, the compounds having the following physical data were obtained.
(9β,11α,13E)-9-Chloro-11,16-dihydroxy-17,17-propano-19-methyl-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid Methyl Ester
More Polar
TLC Rf 0.65 (hexane:ethyl acetate=1:1);
NMR (CDCl3): δ 7.95 (2H, d, J=8.5 Hz), 7.26 (2H, d, J=8.5 Hz), 5.60 (1H, ddd, J=15, 8.6, 5.2 Hz), 5.40 (1H, dd, J=15, 7.7 Hz), 4.09 (2H, m), 3.90 (3H, s), 3.61 (1H, dd, J=10, 1.8 Hz), 2.86 (1H, bs), 2.78 (2H, t, J=7.8 Hz), 2.38-1.62 (15H, m), 1.54 (1H, dd, J=14, 6.8 Hz), 1.33 (1H, dd, J=14, 6.5 Hz), 0.92 (3H, d, J=6.2 Hz), 0.91 (3H, d, J=6.1 Hz).
(9β,11α,13E)-9-Chloro-11,16-dihydroxy-17,17-propano-1,6-(p-phenylene)-2,3,4,5,20-pentanorprost-13-enoic Acid Methyl Ester
More Polar
TLC: Rf 0.45 (toluene:isopropylalcohol=9:1);
NMR (CDCl3): δ 7.95 (2H, d, J=8.0 Hz), 7.25 (2H, d, J=8.0 Hz), 5.58 (1H, ddd, J=15.4, 8.0, 5.6 Hz), 5.38 (1H, dd, J=15.4, 8.0 Hz), 4.16-4.02 (2H, m), 3.90 (3H, s), 3.53 (1H, dd, J=10.0, 2.2 Hz), 2.78 (2H, t, J=7.8 Hz), 2.37-1.32 (18H, m), 0.91 (3H, t, J=7.4 Hz).
(9β,11α,13E)-9-Chloro-11,16-dihydroxy-17,17-propano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13,19-dienoic Acid Methyl Ester
More Polar
TLC Rf 0.39 (toluene:isopropylalcohol=9:1);
NMR (CDCl3): δ 7.95 (2H, d, J=8.4 Hz), 7.25 (2H, d, J=8.4 Hz), 5.93 (1H, ddt, J=17.2, 9.8, 7.4 Hz), 5.56 (1H, ddd, J=15.4, 8.0, 5.6 Hz), 5.37 (1H, dd, J=15.4, 8.0 Hz), 5.15-5.06 (2H, m), 4.15-4.02 (2H, m), 3.90 (3H, s), 3.52 (1H, dd, J=10.2, 2.6 Hz), 2.77 (2H, t, J=7.6 Hz), 2.42-1.60 (18H, m).
(9β,11α,13E) -9-Chloro-11,16-dihydroxy-17,17-propano-19,20-methano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic Acid
To a solution of the compound prepared in example 8 (10 mg) in methanol (1 ml), was added 2N aqueous solution of sodium hydroxide (0.5 ml) and the mixture was stirred at room temperature for 4 hours. The mixture was acidified by addition of 1N hydrochloric acid and the mixture was extracted with ethyl acetate. The organic layer was washed, dried, concentrated in vacuo. The residue was purified by column chromatography on silica gel (Wakogel C-200, 1 g, hexane/ethyl acetate=2/1→1/1) to give the title compound (9.3 mg) having the following physical data
TLC: Rf 0.26 (hexane:ethyl acetate=1:2);
NMR (CDCl3): δ 8.01 (2H, d, J=8.0 Hz), 7.28 (2H, d, J=8.0 Hz), 5.57 (1H, m), 5.39 (1H, m), 4.38 (3H, br), 4.10 (2H, m), 3.70 (1H, bd, J=8.4 Hz), 2.79 (2H, t, J=7.6 Hz), 2.40-1.62 (14H, m), 1.52 (1H, dd, J=14, 6.6 Hz), 1.36 (1H, dd, J=14, 6.4 Hz), 0.78 (1H, m), 0.49 (2H, m), 0.10 (2H, m).
By the same procedure as example 9, using the compounds prepared in example 8(a)-8(c), the compounds having the following physical data were obtained.
(9β,11α,13E)-9-Chloro-11,16-dihydroxy-17,17-propano-19-methyl-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic acid
TLC Rf 0.33 (hexane ethyl acetate=1:2);
NMR (CDCl): δ 8.00 (2H, d, J=8.2 Hz), 7.27 (2H., d, J=8.2 Hz), 5.60 (1H, ddd, J=15, 8.8, 5.2 Hz), 5.42 (1H, dd, J=15, 7.8 Hz), 4.12 (2H, m), 3.69 (3H, br), 3.63 (1H, dd, J=10, 1.8 Hz), 2.79 (2H, t, J=7.9 Hz), 2.38-1.62 (15H, m), 1.54 (1H, dd, J=14, 6.8 Hz), 1.33 (1H, dd, J=14, 6.4 Hz), 0.91 (3H, d, J=6.6 Hz, 0.90 (3H, d, J=6.2 Hz).
(9β,11α,13E)-9-Chloro-11,16-dihydroxy-17,17-propano-1,6-(p-phenylene)-2,3,4,5,20-pentanorprost-13-enoic acid
TLC: Rf 0.14 (hexane:ethyl acetate=1:1);
NMR (CDCl3): δ 8.00 (2H, d, J=8.0 Hz), 7.27 (2H, cl, J=18.0 Hz), 5.58 (1H, ddd, J=15.4, 8.4, 6.6 Hz), 5.40 (1H, dd, J=15.4, 8.2 Hz), 4.40-3.00 (3H, br), 4.18-4.03 (2H, m), 3.56 (1H, dd, J=10.2, 2.2 Hz), 2.79 (2H, t, J=7.6 Hz), 2.38-1.30 (16H, m), 0.91 (3H, t, J=7.8 Hz).
(9β,11α,13E) -9-Chloro-11,16-dihydroxy-17,17-propano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13,19-dienoic acid
TLC: Rf 0.15 (hexane:ethyl acetate=1:1);
NMR (CDCl3): δ 8.00 (2H, d, J=8.0 Hz), 7.27 (2H, d, J=18.0 Hz), 5.93 (1H, ddt, J=17.4, 10.0, 7.2 Hz), 5.65-5.50 (1H, m), 5.38 (1H, dd, J=15.4, 8.4 Hz), 5.15-5.06 (2H, m), 4.80-3.60 (3H, br), 4.18-4.00 (2H, m), 3.55 (1H, bd, J=8.4 Hz), 2.78 (2H, t, J=7.6 Hz), 2.41-1.60 (16H, m).
The following compounds were admixed in the conventional method, dried, and was added microcrystalline cellulose thereon to weigh 10 g in total, mixed until homogeneous and punched out in conventional method to obtain 100 tablets each containing 30 μg of active ingredient.
The solution of (11α,13E)-9-oxo-11,16-dihydroxy-17,17-propano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic
| acid (3 mg) in ethanol | 10 | ml | ||
| Magnesium stearate | 100 | mg | ||
| silicon dioxide | 20 | mg | ||
| talc | 10 | mg | ||
| Carboxymethylcellulose calcium | 200 | mg | ||
| Microcrystalline cellulose | 5.0 | g | ||
Claims (16)
2. A compound according to claim 1, wherein A is a benzene ring.
3. A compound according to claim 1, wherein R2 is C1-4 alkylene or C2-4 alkenylene.
4. A compound according to claim 1, wherein R2is —S—C1-4 alkylene, —S—C2-4 alkenylene.
5. A compound according to claim 1, wherein R2 is C1-4 alkylene-S—.
6. A compound according to claim 3, which is
(1) (11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic acid,
(2) (11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13,19-dienoic acid,
(3) (11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-19,20-methano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic acid,
(4) (11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-19-methyl-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic acid,
(5) (11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano—1,6-(p-phenylene)-2,3,4,5,20-pentanorprost-13-enoic acid,
(6) (9β,11α,13E)-9-Chloro-11,16-dihydroxy-17,17-propano-19,20-methano—1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic acid,
(7) (9β, 11α,13E)-9-Chloro-11,16-dihydroxy-17,17-propano-19-methyl-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic acid,
(8) (9β,11α,13E)-9-Chloro-11,16-dihydroxy-17,17propano-1,6-(p-phenylene)-2,3,4,5,20-pentanorprost-13-enoic acid or
(9) (9β,11α,13E)-9-Chloro-11,16-dihydroxy-17,17-propano-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13,19-dienoic acid or a methyl ester thereof.
7. A compound according to claim 4, which is
(1) (11α,8Z, 13E)-9-Acetyloxy-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5,20-pentanorprost-8,13-dienoic acid methyl ester,
(2) (11α,8Z, 13E)-9-Acetyloxy-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-8,13-dienoic acid methyl ester,
(3) (11α,8Z, 13E)-9-Acetyloxy-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-8,13,19-trienoic acid methyl ester,
(4) (11α,8Z, 13E)-9-Acetyloxy-11,16-dihydroxy-19-methyl-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-8,13-dienoic acid methyl ester or
(5) (11α,8Z, 13E)-9-Acetyloxy-11,16-dihydroxy-17,17-propano-19,20-methano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-8,13-dienoic acid methyl ester.
8. A compound according to claim 4, which is
(1) (11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5,20-pentanorprost-13-enoic acid,
(2) (11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic acid,
(3) (11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13,19-dienoic acid,
(4) (11α,13E)-9-Oxo-11,16-dihydroxy-19-methyl-17,17-propano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic acid or
(5) (11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-19,20-methano-7-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic acid
or a methyl ester thereof.
9. A compound according to claim 5, which is
(1) (11α,13E)-9-Oxo-11,16-dihydroxy-17,17-propano-6-thia-1,6-(p-phenylene)-2,3,4,5-tetranorprost-13-enoic acid or a methyl ester thereof.
10. A compound according to claim 2, wherein R2 is C1-4 alkylene or C2-4 alkenylene.
11. A compound according to claim 2, wherein R2 is C1-4 alkylene-S—.
(wherein R30 is oxo, methylene or halogen atom and the other symbols are as defined in claim 1)
(wherein R12 is C1-6 alkyl and the other symbols are as defined above)
to hydrolysis using an enzyme or hydrolysis under alkaline conditions.
(wherein R30 is oxo, methylene or halogen atom, and the other symbols are as defined in claim 1)
(wherein all symbols are as defined above)
and a compound of formula (II)
(wherein all symbols are as defined above).
(wherein R40 is hydrogen or hydroxy, R12 is C1-6 and R30 is oxo, methylene or halogen atom, and the other symbols are as defined in claim 1) characterized by subjecting to deprotection under acidic conditions a compound of formula (III)
(wherein R41 is hydrogen atom hydroxy protected by a group which can be removable under acidic conditions, R60 is a protective group for hydroxy which may be removable under acidic conditions, and the other symbols are as defined above).
(wherein R12 is C1-6 and R30 is oxo, methylene or halogen atom, R42 is C1-4 alkoxy, and the other symbls are as defined in claim 1)
(wherein all symbols are as defined above).
(wherein R12 is C1-6 alkyl, R32 is as defined in claim 1, and the other symbols are as defined in claim 1) characterized by subjecting to deprotection under acidic conditions a compound of formula (IV)
(wherein, R43 is hydrogen atom, hydroxy protected by a group which may be removable under acidic conditions or C1-4 alkoxy, R60 is a protective Group for hydroxy which may be removable under acidic conditions, and the other symbols are as defined above).
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP36624497 | 1997-12-25 | ||
| JP9-366244 | 1997-12-25 | ||
| PCT/JP1998/005863 WO1999033794A1 (en) | 1997-12-25 | 1998-12-24 | φ-CYCLOALKYL-PROSTAGLANDIN E2 DERIVATIVES |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US6262293B1 true US6262293B1 (en) | 2001-07-17 |
Family
ID=18486293
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/582,348 Expired - Fee Related US6262293B1 (en) | 1997-12-25 | 1998-12-24 | ω-Cycloalkly-prostaglandin e2 derivatives |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US6262293B1 (en) |
| EP (1) | EP1042283B1 (en) |
| KR (1) | KR20010033538A (en) |
| AT (1) | ATE260254T1 (en) |
| DE (1) | DE69821987T2 (en) |
| ES (1) | ES2216336T3 (en) |
| WO (1) | WO1999033794A1 (en) |
Cited By (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6376533B1 (en) * | 2000-10-20 | 2002-04-23 | Allergan Sales, Inc. | Omega-cycloalkyl 17-heteroaryl prostaglandin E2 analogs as EP2-receptor agonists |
| US6586462B2 (en) | 2000-10-20 | 2003-07-01 | Allergan, Inc. | ω-Cycloalkyl 17-heteroaryl prostaglandin E2 analogs as EP2-receptor agonists |
| US6639111B2 (en) * | 2000-01-05 | 2003-10-28 | Ono Pharmaceutical Co., Ltd. | 5-thia-ω-(substituted phenyl)-prostaglandin E alcohols, process for preparing the alcohols and pharmaceutical preparations containing the same as the active ingredient |
| WO2004073591A2 (en) * | 2003-02-24 | 2004-09-02 | Bayer Healthcare Ag | Diagnostics and therapeutics for diseases associated with g-protein coupled receptor prostaglandin e2 ep2 (prostaglandin e2 ep2) |
| US20060252839A1 (en) * | 2002-03-27 | 2006-11-09 | Roberts Stanley M | Pharmaceutical compositions |
| US20070270489A1 (en) * | 2003-07-25 | 2007-11-22 | Ono Pharmaceutical Co., Ltd. | Remedy for Cartilage-Related Diseases |
| US20100099728A1 (en) * | 2008-10-17 | 2010-04-22 | Xenon Pharmaceuticals Inc. | Spiro-oxindole compounds and their use as therapeutic agents |
| US20100125072A1 (en) * | 2005-04-11 | 2010-05-20 | Xenon Pharmaceuticals Inc. | Spiro-oxindole compounds and their uses as therapeutic agents |
| US20100137299A1 (en) * | 2008-10-17 | 2010-06-03 | Xenon Pharmaceuticals Inc. | Spiro-oxindole compounds and their use as therapeutic agents |
| US20100160362A1 (en) * | 2006-10-12 | 2010-06-24 | Xenon Pharmaceuticals Inc. | Spiro (furo [3, 2-c] pyridine-3-3' -indol) -2' (1'h)-one derivatives and related compounds for the treatment of sodium-channel mediated diseases, such as pain |
| US20100331386A1 (en) * | 2009-06-29 | 2010-12-30 | Xenon Pharmaceuticals Inc. | Enantiomers of spiro-oxindole compounds and their uses as therapeutic agents |
| US20110034500A1 (en) * | 2005-04-11 | 2011-02-10 | Xenon Pharmaceuticals Inc. | Spiroheterocyclic compounds and their uses as therapeutic agents |
| US20110087027A1 (en) * | 2009-10-14 | 2011-04-14 | Xenon Pharmaceuticals Inc. | Synthetic methods for spiro-oxindole compounds |
| US20110086899A1 (en) * | 2009-10-14 | 2011-04-14 | Xenon Pharmaceuticals Inc. | Pharmaceutical compositions for oral administration |
| US20110190286A1 (en) * | 2010-01-29 | 2011-08-04 | Allergan, Inc. | Therapeutically active cyclopentanes |
| WO2011106729A2 (en) | 2010-02-26 | 2011-09-01 | Xenon Pharmaceuticals Inc. | Pharmaceutical compositions of spiro-oxindole compound for topical administration and their use as therapeutic agents |
| US8466188B2 (en) | 2006-10-12 | 2013-06-18 | Xenon Pharmaceuticals Inc. | Use of spiro-oxindole compounds as therapeutic agents |
| US8476471B2 (en) | 2009-07-13 | 2013-07-02 | Irix Pharmaceuticals, Inc. | Synthesis of prostanoids |
| WO2014152229A2 (en) | 2013-03-14 | 2014-09-25 | Celtaxsys, Inc. | Inhibitors of leukotriene a4 hydrolase |
| US9487535B2 (en) | 2012-04-12 | 2016-11-08 | Xenon Pharmaceuticals Inc. | Asymmetric syntheses for spiro-oxindole compounds useful as therapeutic agents |
| US9682033B2 (en) | 2015-02-05 | 2017-06-20 | Teva Pharmaceuticals International Gmbh | Methods of treating postherpetic neuralgia with a topical formulation of a spiro-oxindole compound |
| EP3205644A1 (en) | 2005-12-29 | 2017-08-16 | Celtaxsys Inc. | Diamine derivatives as inhibitors of leukotriene a4 hydrolase |
| US9968716B2 (en) | 2013-10-15 | 2018-05-15 | Ono Pharmaceutical Co., Ltd. | Drug-eluting stent graft |
| WO2018107153A1 (en) | 2016-12-09 | 2018-06-14 | Celtaxsys, Inc. | Inhibitors of leukotriene a4 hydrolase |
| WO2018107167A1 (en) | 2016-12-09 | 2018-06-14 | Celtaxsys, Inc. | Pendant amines and derivatives as inhibitors of leukotriene a4 hydrolase |
| WO2018107158A1 (en) | 2016-12-09 | 2018-06-14 | Celtaxsys, Inc. | Monamine and monoamine derivatives as inhibitors of leukotriene a4 hydrolase |
| US10100060B2 (en) | 2016-06-16 | 2018-10-16 | Xenon Pharmaceuticals Inc. | Asymmetric synthesis of funapide |
| US10100028B2 (en) | 2013-09-30 | 2018-10-16 | Patheon Api Services Inc. | Synthesis routes for prostaglandins and prostaglandin intermediates using metathesis |
| US10385045B2 (en) | 2015-07-23 | 2019-08-20 | Ono Pharmaceutical Co., Ltd. | Compound having EP2 agonist activity |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6235780B1 (en) * | 1998-07-21 | 2001-05-22 | Ono Pharmaceutical Co., Ltd. | ω-cycloalkyl-prostaglandin E1 derivatives |
| KR20010108316A (en) | 1999-03-05 | 2001-12-07 | 데이비드 엠 모이어 | C16 unsaturated fp-selective prostaglandins analogs |
| US20020013294A1 (en) | 2000-03-31 | 2002-01-31 | Delong Mitchell Anthony | Cosmetic and pharmaceutical compositions and methods using 2-decarboxy-2-phosphinico derivatives |
| US20020172693A1 (en) | 2000-03-31 | 2002-11-21 | Delong Michell Anthony | Compositions and methods for treating hair loss using non-naturally occurring prostaglandins |
| JP2002027998A (en) * | 2000-09-29 | 2002-01-29 | Shionogi & Co Ltd | Method for measuring activity for regulating degeneration of nerve cell |
| JP2006021998A (en) * | 2002-07-18 | 2006-01-26 | Ono Pharmaceut Co Ltd | Dysmenorrhea-treating agent containing ep2 agonist as active ingredient |
| US7547715B2 (en) | 2002-10-10 | 2009-06-16 | Ono Pharmaceutical Co., Ltd. | Endogenous repair factor production accelerator |
| ATE394372T1 (en) | 2003-03-03 | 2008-05-15 | Serono Lab | G-LACTAM DERIVATIVES AS PROSTAGLAND INAGONISTS |
| JP2005511607A (en) * | 2003-07-10 | 2005-04-28 | アラーガン、インコーポレイテッド | Wireless microphone communication system |
| US7465755B2 (en) | 2003-07-18 | 2008-12-16 | Laboratoires Serono Sa | Hydrazide derivatives as prostaglandin receptors modulators |
| WO2006043655A1 (en) | 2004-10-22 | 2006-04-27 | Ono Pharmaceutical Co., Ltd. | Medicinal composition for inhalation |
| KR101320810B1 (en) | 2005-06-03 | 2013-10-21 | 오노 야꾸힝 고교 가부시키가이샤 | Agent for regeneration and/or protection of nerves |
| EP2913047B1 (en) | 2012-10-29 | 2019-05-08 | Cardio Incorporated | Pulmonary disease-specific therapeutic agent |
| WO2023127855A1 (en) * | 2021-12-28 | 2023-07-06 | 大内新興化学工業株式会社 | Cyclopentenone derivative and method for producing same |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4132738A (en) | 1978-02-23 | 1979-01-02 | Miles Laboratories, Inc. | Preparation of 15-deoxy-16-hydroxyprostaglandins |
| US4275224A (en) | 1978-02-23 | 1981-06-23 | Miles Laboratories, Inc. | 15-Deoxy-16-hydroxy prostaglandins |
-
1998
- 1998-12-24 ES ES98961522T patent/ES2216336T3/en not_active Expired - Lifetime
- 1998-12-24 WO PCT/JP1998/005863 patent/WO1999033794A1/en not_active Application Discontinuation
- 1998-12-24 AT AT98961522T patent/ATE260254T1/en not_active IP Right Cessation
- 1998-12-24 US US09/582,348 patent/US6262293B1/en not_active Expired - Fee Related
- 1998-12-24 KR KR1020007007046A patent/KR20010033538A/en not_active Application Discontinuation
- 1998-12-24 EP EP98961522A patent/EP1042283B1/en not_active Expired - Lifetime
- 1998-12-24 DE DE69821987T patent/DE69821987T2/en not_active Expired - Fee Related
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4132738A (en) | 1978-02-23 | 1979-01-02 | Miles Laboratories, Inc. | Preparation of 15-deoxy-16-hydroxyprostaglandins |
| JPS54115351A (en) | 1978-02-23 | 1979-09-07 | Miles Lab | 155deoxyy166hydroxyprostaglandin |
| US4275224A (en) | 1978-02-23 | 1981-06-23 | Miles Laboratories, Inc. | 15-Deoxy-16-hydroxy prostaglandins |
Non-Patent Citations (2)
Cited By (49)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6639111B2 (en) * | 2000-01-05 | 2003-10-28 | Ono Pharmaceutical Co., Ltd. | 5-thia-ω-(substituted phenyl)-prostaglandin E alcohols, process for preparing the alcohols and pharmaceutical preparations containing the same as the active ingredient |
| US6376533B1 (en) * | 2000-10-20 | 2002-04-23 | Allergan Sales, Inc. | Omega-cycloalkyl 17-heteroaryl prostaglandin E2 analogs as EP2-receptor agonists |
| US6586462B2 (en) | 2000-10-20 | 2003-07-01 | Allergan, Inc. | ω-Cycloalkyl 17-heteroaryl prostaglandin E2 analogs as EP2-receptor agonists |
| US20060252839A1 (en) * | 2002-03-27 | 2006-11-09 | Roberts Stanley M | Pharmaceutical compositions |
| US7759399B2 (en) * | 2002-03-27 | 2010-07-20 | Crawford Healthcare Holdings Limited | Pharmaceutical compositions |
| WO2004073591A2 (en) * | 2003-02-24 | 2004-09-02 | Bayer Healthcare Ag | Diagnostics and therapeutics for diseases associated with g-protein coupled receptor prostaglandin e2 ep2 (prostaglandin e2 ep2) |
| WO2004073591A3 (en) * | 2003-02-24 | 2004-09-23 | Bayer Healthcare Ag | Diagnostics and therapeutics for diseases associated with g-protein coupled receptor prostaglandin e2 ep2 (prostaglandin e2 ep2) |
| US20070270489A1 (en) * | 2003-07-25 | 2007-11-22 | Ono Pharmaceutical Co., Ltd. | Remedy for Cartilage-Related Diseases |
| US8106087B2 (en) | 2005-04-11 | 2012-01-31 | Xenon Pharmaceuticals Inc. | Spiro-oxindole compounds and their uses as therapeutic agents |
| US20100125072A1 (en) * | 2005-04-11 | 2010-05-20 | Xenon Pharmaceuticals Inc. | Spiro-oxindole compounds and their uses as therapeutic agents |
| US20110034500A1 (en) * | 2005-04-11 | 2011-02-10 | Xenon Pharmaceuticals Inc. | Spiroheterocyclic compounds and their uses as therapeutic agents |
| EP3205644A1 (en) | 2005-12-29 | 2017-08-16 | Celtaxsys Inc. | Diamine derivatives as inhibitors of leukotriene a4 hydrolase |
| US20100160362A1 (en) * | 2006-10-12 | 2010-06-24 | Xenon Pharmaceuticals Inc. | Spiro (furo [3, 2-c] pyridine-3-3' -indol) -2' (1'h)-one derivatives and related compounds for the treatment of sodium-channel mediated diseases, such as pain |
| US8466188B2 (en) | 2006-10-12 | 2013-06-18 | Xenon Pharmaceuticals Inc. | Use of spiro-oxindole compounds as therapeutic agents |
| US8263606B2 (en) | 2008-10-17 | 2012-09-11 | Xenon Pharmaceuticals Inc. | Spiro-oxindole compounds and their use as therapeutic agents |
| US20100137299A1 (en) * | 2008-10-17 | 2010-06-03 | Xenon Pharmaceuticals Inc. | Spiro-oxindole compounds and their use as therapeutic agents |
| US9458178B2 (en) | 2008-10-17 | 2016-10-04 | Xenon Pharmaceuticals Inc. | Spiro-oxindole compounds and their use as therapeutic agents |
| US8916580B2 (en) | 2008-10-17 | 2014-12-23 | Xenon Pharmaceuticals Inc. | Spiro-oxindole compounds and their use as therapeutic agents |
| US20110112162A9 (en) * | 2008-10-17 | 2011-05-12 | Xenon Pharmaceuticals Inc. | Spiro-oxindole compounds and their use as therapeutic agents |
| US8415370B2 (en) | 2008-10-17 | 2013-04-09 | Xenon Pharmaceuticals Inc. | Spiro-oxindole compounds and their uses as therapeutic agents |
| US8101647B2 (en) | 2008-10-17 | 2012-01-24 | Xenon Pharmaceuticals Inc. | Spiro-oxindole compounds and their use as therapeutic agents |
| US20100099728A1 (en) * | 2008-10-17 | 2010-04-22 | Xenon Pharmaceuticals Inc. | Spiro-oxindole compounds and their use as therapeutic agents |
| US20100331386A1 (en) * | 2009-06-29 | 2010-12-30 | Xenon Pharmaceuticals Inc. | Enantiomers of spiro-oxindole compounds and their uses as therapeutic agents |
| US9480677B2 (en) | 2009-06-29 | 2016-11-01 | Xenon Pharmaceuticals Inc. | Enantiomers of spiro-oxindole compounds and their uses as therapeutic agents |
| US8883840B2 (en) | 2009-06-29 | 2014-11-11 | Xenon Pharmaceuticals Inc. | Enantiomers of spiro-oxindole compounds and their uses as therapeutic agents |
| US8450358B2 (en) | 2009-06-29 | 2013-05-28 | Xenon Pharmaceuticals Inc. | Enantiomers of spiro-oxindole compounds and their uses as therapeutic agents |
| US8476471B2 (en) | 2009-07-13 | 2013-07-02 | Irix Pharmaceuticals, Inc. | Synthesis of prostanoids |
| US8742109B2 (en) | 2009-10-14 | 2014-06-03 | Xenon Pharmaceuticals Inc. | Synthetic methods for spiro-oxindole compounds |
| US20110086899A1 (en) * | 2009-10-14 | 2011-04-14 | Xenon Pharmaceuticals Inc. | Pharmaceutical compositions for oral administration |
| US20110087027A1 (en) * | 2009-10-14 | 2011-04-14 | Xenon Pharmaceuticals Inc. | Synthetic methods for spiro-oxindole compounds |
| US9695185B2 (en) | 2009-10-14 | 2017-07-04 | Xenon Pharmaceuticals Inc. | Synthetic methods for spiro-oxindole compounds |
| US8445696B2 (en) | 2009-10-14 | 2013-05-21 | Xenon Pharmaceuticals Inc. | Synthetic methods for spiro-oxindole compounds |
| WO2011047173A2 (en) | 2009-10-14 | 2011-04-21 | Xenon Pharmaceuticals Inc. | Pharmaceutical compositions for oral administration |
| US9260446B2 (en) | 2009-10-14 | 2016-02-16 | Xenon Pharmaceuticals Inc. | Synthetic methods for spiro-oxindole compounds |
| US20110190286A1 (en) * | 2010-01-29 | 2011-08-04 | Allergan, Inc. | Therapeutically active cyclopentanes |
| US8299068B2 (en) * | 2010-01-29 | 2012-10-30 | Allergan, Inc. | Therapeutically active cyclopentanes |
| WO2011106729A2 (en) | 2010-02-26 | 2011-09-01 | Xenon Pharmaceuticals Inc. | Pharmaceutical compositions of spiro-oxindole compound for topical administration and their use as therapeutic agents |
| US9504671B2 (en) | 2010-02-26 | 2016-11-29 | Xenon Pharmaceuticals Inc. | Pharmaceutical compositions of spiro-oxindole compound for topical administration and their use as therapeutic agents |
| EP3266444A1 (en) | 2010-02-26 | 2018-01-10 | Xenon Pharmaceuticals Inc. | Pharmaceutical compositions of spiro-oxindole compound for topical administration and their use as therapeutic agents |
| US9487535B2 (en) | 2012-04-12 | 2016-11-08 | Xenon Pharmaceuticals Inc. | Asymmetric syntheses for spiro-oxindole compounds useful as therapeutic agents |
| WO2014152229A2 (en) | 2013-03-14 | 2014-09-25 | Celtaxsys, Inc. | Inhibitors of leukotriene a4 hydrolase |
| US10100028B2 (en) | 2013-09-30 | 2018-10-16 | Patheon Api Services Inc. | Synthesis routes for prostaglandins and prostaglandin intermediates using metathesis |
| US9968716B2 (en) | 2013-10-15 | 2018-05-15 | Ono Pharmaceutical Co., Ltd. | Drug-eluting stent graft |
| US9682033B2 (en) | 2015-02-05 | 2017-06-20 | Teva Pharmaceuticals International Gmbh | Methods of treating postherpetic neuralgia with a topical formulation of a spiro-oxindole compound |
| US10385045B2 (en) | 2015-07-23 | 2019-08-20 | Ono Pharmaceutical Co., Ltd. | Compound having EP2 agonist activity |
| US10100060B2 (en) | 2016-06-16 | 2018-10-16 | Xenon Pharmaceuticals Inc. | Asymmetric synthesis of funapide |
| WO2018107167A1 (en) | 2016-12-09 | 2018-06-14 | Celtaxsys, Inc. | Pendant amines and derivatives as inhibitors of leukotriene a4 hydrolase |
| WO2018107158A1 (en) | 2016-12-09 | 2018-06-14 | Celtaxsys, Inc. | Monamine and monoamine derivatives as inhibitors of leukotriene a4 hydrolase |
| WO2018107153A1 (en) | 2016-12-09 | 2018-06-14 | Celtaxsys, Inc. | Inhibitors of leukotriene a4 hydrolase |
Also Published As
| Publication number | Publication date |
|---|---|
| DE69821987D1 (en) | 2004-04-01 |
| ES2216336T3 (en) | 2004-10-16 |
| ATE260254T1 (en) | 2004-03-15 |
| KR20010033538A (en) | 2001-04-25 |
| EP1042283A1 (en) | 2000-10-11 |
| EP1042283B1 (en) | 2004-02-25 |
| WO1999033794A1 (en) | 1999-07-08 |
| DE69821987T2 (en) | 2004-12-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6262293B1 (en) | ω-Cycloalkly-prostaglandin e2 derivatives | |
| US6576785B1 (en) | ω-cycloalkyl-prostaglandin E2 derivatives | |
| US6235780B1 (en) | ω-cycloalkyl-prostaglandin E1 derivatives | |
| US6462081B1 (en) | 5-thia-ω-substituted phenyl-prostaglandin E derivatives, process for producing the same and drugs containing the same as the active ingredient | |
| EP0855389B1 (en) | A 3,7-dithiaprostanoic acid derivative | |
| US6586468B1 (en) | ω-substituted phenyl-prostaglandin E derivatives and drugs containing the same as the active ingredient | |
| US6288119B1 (en) | 11,15-O-dialkylprostaglandin E derivatives, process for producing the same, and drugs containing the same as the active ingredient | |
| EP0985663A1 (en) | A 3.7-dithiaprostanoic acid derivative | |
| JP3162668B2 (en) | ω-cycloalkyl-prostaglandin E2 derivative | |
| EP0122019B1 (en) | A prostaglandin analogue | |
| JP2001527063A (en) | ω-cycloalkyl-prostaglandin E2 derivative | |
| JP4029251B2 (en) | ω-cycloalkyl-prostaglandin E2 derivative | |
| JP4123568B2 (en) | 6-oxo-PGE1 compounds, processes for their preparation and prostaglandin E2 receptor agonists containing them as active ingredients | |
| JP2000001472A (en) | 3,7-dithiaprostanoic acid, its production and pharmaceutical agent containing the same | |
| JP3703004B2 (en) | 5-thia-ω-substituted phenyl-prostaglandin E derivatives | |
| JP2000095755A (en) | Omega-cycloalkyl-prostaglandin e1 derivative, its production, and medicinal composition containing the derivative as active ingredient |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: ONO PHARMACEUTICAL CO., LTD., JAPAN Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:TANI, KOUSUKE;OHUCHIDA, SHUICHI;REEL/FRAME:010951/0994 Effective date: 20000612 |
|
| CC | Certificate of correction | ||
| FEPP | Fee payment procedure |
Free format text: PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY |
|
| REMI | Maintenance fee reminder mailed | ||
| LAPS | Lapse for failure to pay maintenance fees | ||
| STCH | Information on status: patent discontinuation |
Free format text: PATENT EXPIRED DUE TO NONPAYMENT OF MAINTENANCE FEES UNDER 37 CFR 1.362 |
|
| FP | Lapsed due to failure to pay maintenance fee |
Effective date: 20050717 |