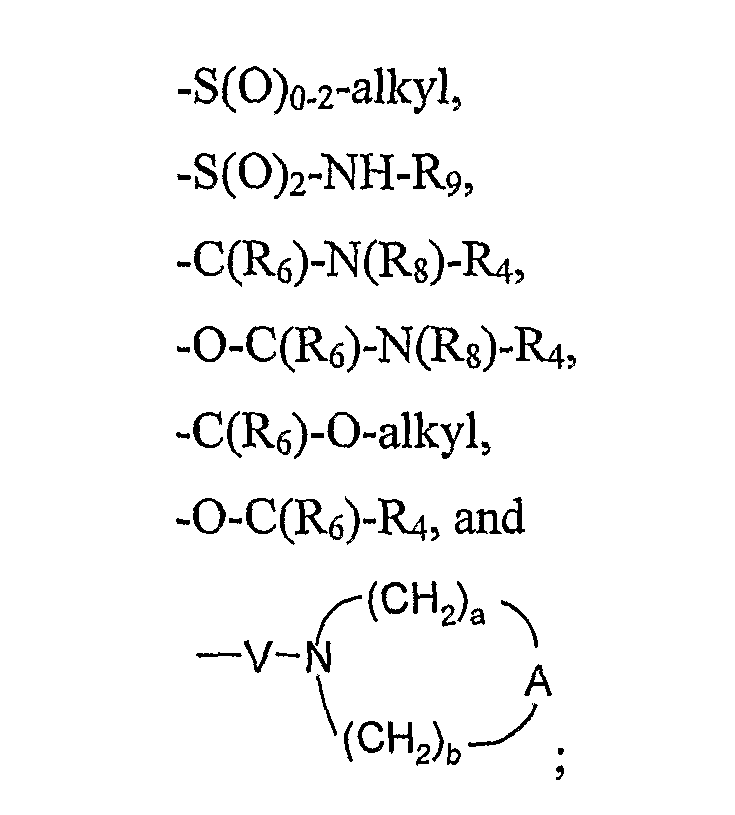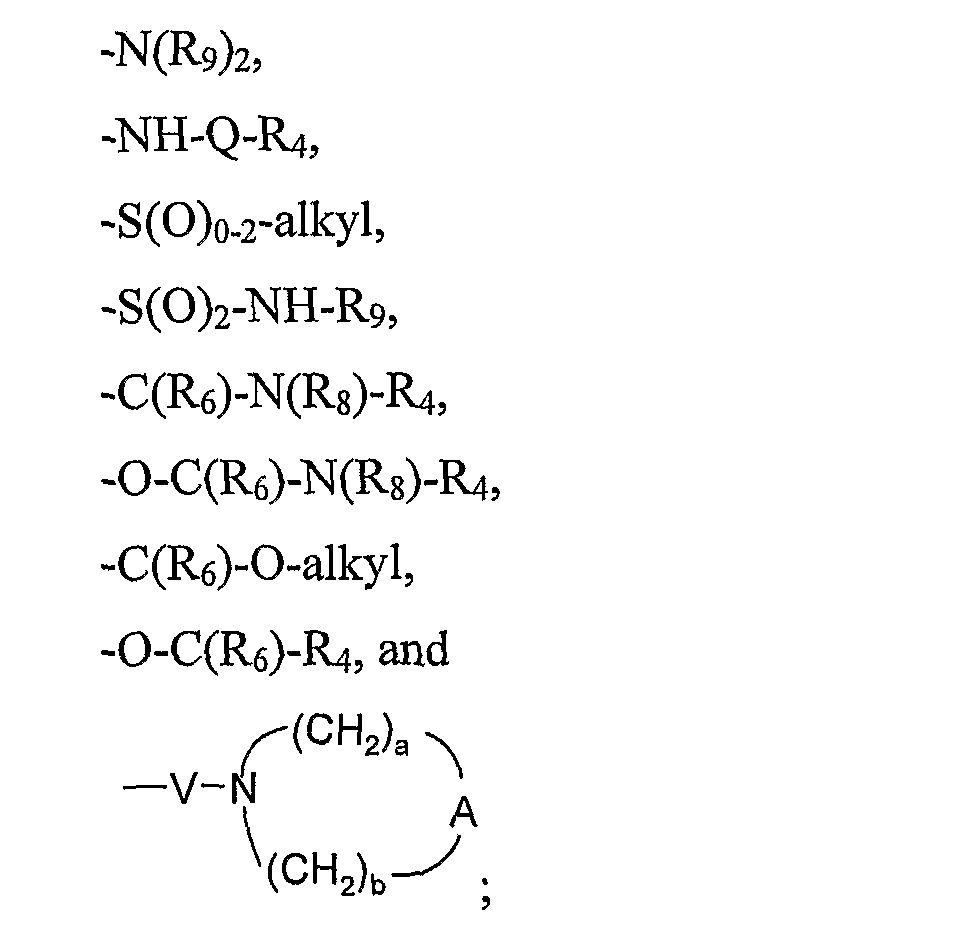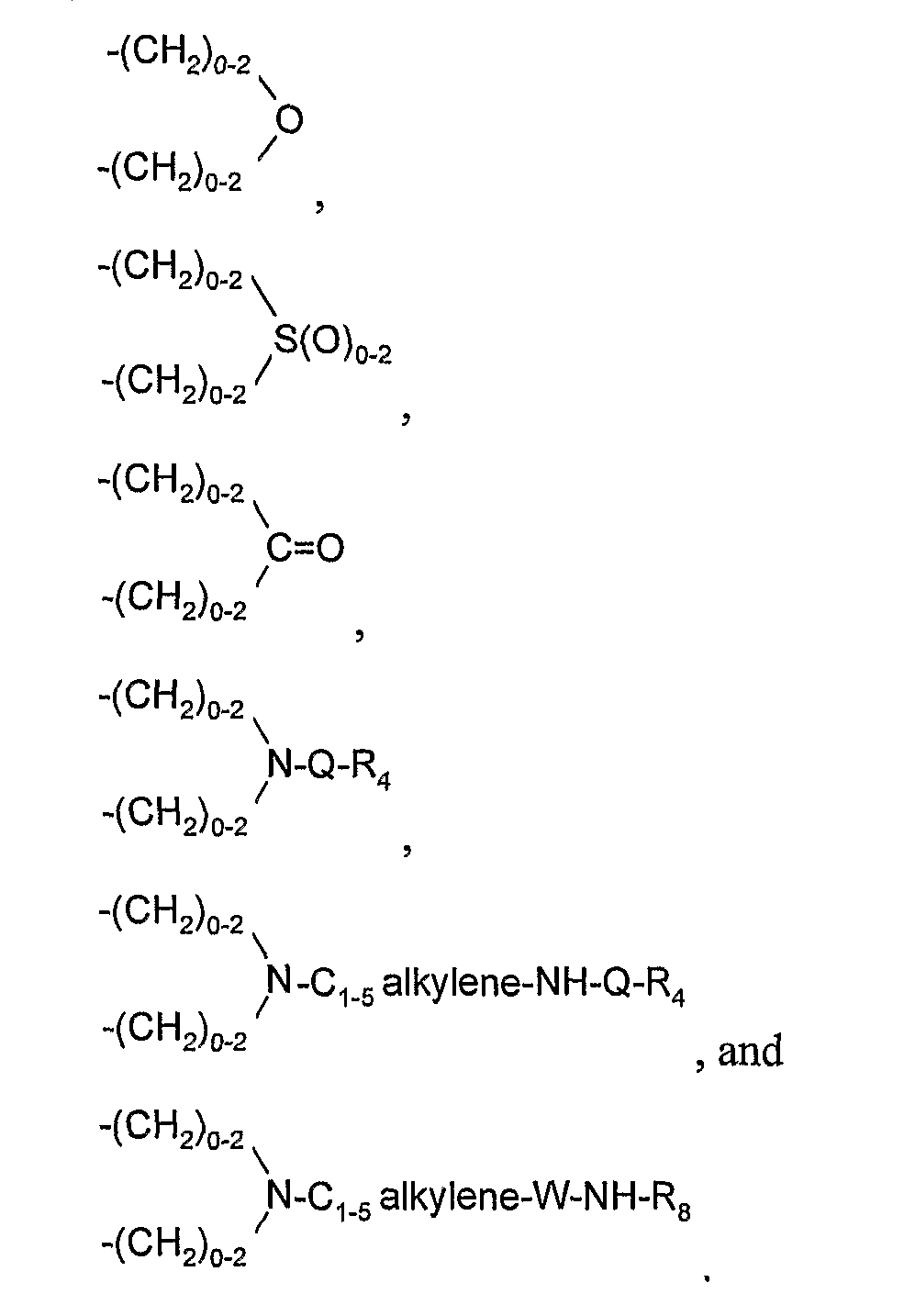WO2006107851A1 - 1-substituted pyrazolo (3,4-c) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases - Google Patents
1-substituted pyrazolo (3,4-c) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases Download PDFInfo
- Publication number
- WO2006107851A1 WO2006107851A1 PCT/US2006/012263 US2006012263W WO2006107851A1 WO 2006107851 A1 WO2006107851 A1 WO 2006107851A1 US 2006012263 W US2006012263 W US 2006012263W WO 2006107851 A1 WO2006107851 A1 WO 2006107851A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- alkyl
- hydrogen
- compound
- aryl
- Prior art date
Links
- 0 Cc([n](*)nc12)c1c(C=CCC*)c(N)nc2N Chemical compound Cc([n](*)nc12)c1c(C=CCC*)c(N)nc2N 0.000 description 6
- QWTNZMNLIJJROO-UHFFFAOYSA-N CC(N(CC1)CCC1(Cc([n](C)nc12)c1c1ccccc1nc2N)F)=O Chemical compound CC(N(CC1)CCC1(Cc([n](C)nc12)c1c1ccccc1nc2N)F)=O QWTNZMNLIJJROO-UHFFFAOYSA-N 0.000 description 1
- PKAMNWLCIIOUJK-UHFFFAOYSA-N CCC[n]1nc2c(N)nc3ncccc3c2c1CC1CCOCC1 Chemical compound CCC[n]1nc2c(N)nc3ncccc3c2c1CC1CCOCC1 PKAMNWLCIIOUJK-UHFFFAOYSA-N 0.000 description 1
- OMBXPEFOMWFSCY-UHFFFAOYSA-N CC[n]1nc2c(N)nc(cccc3)c3c2c1CC1(CCC1)O Chemical compound CC[n]1nc2c(N)nc(cccc3)c3c2c1CC1(CCC1)O OMBXPEFOMWFSCY-UHFFFAOYSA-N 0.000 description 1
- WKPGHFLSURHXAI-UHFFFAOYSA-N CC[n]1nc2c(N)nc3ccccc3c2c1CC(CC1)(CCN1C(C)=O)F Chemical compound CC[n]1nc2c(N)nc3ccccc3c2c1CC(CC1)(CCN1C(C)=O)F WKPGHFLSURHXAI-UHFFFAOYSA-N 0.000 description 1
- OYGCSEOMHSDDLY-UHFFFAOYSA-N CC[n]1nc2c(N)nc3ccccc3c2c1CC1CCOCC1 Chemical compound CC[n]1nc2c(N)nc3ccccc3c2c1CC1CCOCC1 OYGCSEOMHSDDLY-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains two hetero rings
- C07D471/04—Ortho-condensed systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D471/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00
- C07D471/12—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, not provided for by groups C07D451/00 - C07D463/00 in which the condensed system contains three hetero rings
- C07D471/14—Ortho-condensed systems
Definitions
- the present invention provides a new class of compounds that are useful in inducing cytokine biosynthesis in animals.
- Such compounds are of the following Formula
- R 1 , R 2 , RA, RB 3 X, Z, and m are as defined below.
- the compounds of Formula I are useful as immune response modifiers due to their ability to induce cytokine biosynthesis (e.g., induces the synthesis of at least one cytokine) and otherwise modulate the immune response when administered to animals. This makes the compounds useful in the treatment of a variety of conditions such as viral diseases and tumors that are responsive to such changes in the immune response.
- the invention further provides pharmaceutical compositions containing an effective amount of a compound of Formula I and methods of inducing cytokine biosynthesis in an animal, treating a viral infection or disease and/or treating a neoplastic disease in an animal by administering an effective amount of a compound of Formula I to the animal.
- the present invention provides a compound of the Formula I:
- Ri is selected from the group consisting of: hydrogen, hydroxy, fluorine, alkoxy,
- Ri is other than hydrogen
- m is an integer from 1 to 5
- RA and R B are each independently selected from the group consisting of: hydrogen, halogen, alkyl, alkenyl, alkoxy, alkylthio, and
- R 8 is selected from the group consisting of hydrogen, alkyl, alkoxyalkylenyl, and arylalkylenyl;
- the present invention provides a compound of the Formula XIII:
- haloalkyl is inclusive of groups that are substituted by one or more halogen atoms, including perfluorinated groups. This is also true of other groups that include the prefix “halo-.” Examples of suitable haloalkyl groups are chloromethyl, trifluoromethyl, and the like.
- heterocyclyl groups include pyrrolidinyl, tetrahydrofuranyl, morpholinyl, thiomorpholinyl, 1,1- dioxothiomorpholinyl, piperidinyl, piperazinyl, thiazolidinyl, imidazolidinyl, isothiazolidinyl, tetrahydropyranyl, quinuclidinyl, homopiperidinyl (azepanyl), 1,4- oxazepanyl, homopiperazinyl (diazepanyl), 1,3-dioxolanyl, aziridinyl, azetidinyl, dihydroisoquinolin-(lH)-yl, octahydroisoquinolin-(lH)-yl, dihydroquinolin-(2H) ⁇ yl, octahydroquinolin-(2H)-yl, dihydro-lH-l
- heterocyclyl includes bicyclic and tricyclic heterocyclic ring systems. Such ring systems include fused and/or bridged rings and spiro rings. Fused rings can include, in addition to a saturated or partially saturated ring, an aromatic ring, for example, a benzene ring. Spiro rings include two rings joined by one spiro atom and three rings joined by two spiro atoms.
- fused aryl ring includes fused carbocyclic aromatic rings or ring systems. Examples of fused aryl rings include benzo, naphtho, fluoreno, and indeno.
- fused heteroaryl ring includes the fused forms of 5 or 6 membered aromatic rings that contain one heteroatom selected from S and N.
- each group is independently selected, whether explicitly stated or not.
- each R 9 group is independently selected for the formula -N(R ⁇ - each R 9 group.
- each R 4 group is independently selected for the formula -N(R ⁇ - each R 9 group.
- each R 4 group is independently selected for the formula -N(R ⁇ - each R 9 group.
- each R 4 group is independently selected for the formula -N(R ⁇ - each R 9 group.
- each R 4 group is independently selected.
- tautomer or tautomeric form
- proton tautomers include interconversions via migration of a proton, such as keto-enol and imine-enamine isomerizations.
- proton migration between the 2 and 3 positions may occur.
- any of its embodiments can be combined with any one or more of the other variables in any of their embodiments and associated with any one of the formulas described herein, as would be understood by one oflskill in the art.
- Each of the resulting combinations of variables is an embodiment of the present invention.
- RA and R B are each independently selected from the group consisting of hydrogen, halogen, alkyl, alkenyl, alkoxy, alkylthio, and -N(R 9 ) 2 ; or when taken together, RA and RB form a fused aryl ring or heteroaryl ring containing one heteroatom selected from the group consisting of N and S wherein the aryl or heteroaryl ring is unsubstituted or substituted by one or more R groups; or when taken together, R A and RB form a fused 5 to 7 membered saturated ring, optionally containing one heteroatom selected from the group consisting of N and S, and unsubstituted or substituted by one or more R groups.
- m is an integer from 1 to 5.
- n is an integer from 1 to 3. In certain embodiments, m is 1.
- X is a bond
- R 1 is selected from the group consisting of hydrogen, hydroxy, fluorine, alkoxy, -N(Rg) 2 , -NH-Q-R 4 , -S(0)o -2 -alkyl, -S(O) 2 -NH-R 9 , -C(Re)-N(Rg)-R 4 , -0-C(Rg)-N(Rs)-R 4 , -C(R 6 )-O-alkyl, -0-C(Re)-R 4 , and
- Rj is fluoro, except where Rj is otherwise defined.
- Ri is selected from the group consisting of -NH 2 , -NH-Q-R 4 , -C(O)-NH 2 , and -C(O)-N(Rs)-R 4 , wherein Q is selected from the group consisting of -C(O)-, -S(O) 2 -, -C(O)-O-, and -C(O)-NH-
- R 8 is selected from the group consisting of hydrogen and alkyl
- R 4 is selected from the group consisting of alkyl and alkoxyalkylenyl, except where R 1 is otherwise defined.
- Z is selected from the group consisting of:
- Z is selected from the group consisting of a bond and C 1-3 alkylene.
- X is a bond
- Ri is hydrogen
- Z is
- Q is selected from the group consisting of -C(O)-, -C(O)-O-, -S(O) 2 -, and -C(Re)-N(R 8 )-.
- R 2 is selected from the group consisting of hydrogen, alkyl, alkoxyalkylenyl, hydroxyalkylenyl, haloalkylenyl, and R 4 -C(ReO-O-Ci -4 alkylenyl.
- R 2 is selected from the group consisting of Cj -4 alkyl, Cj -4 alkyl-O-C 2-4 alkylenyl, and hydroxyC 2-4 alkylenyl.
- R 2 is selected from the group consisting of methyl, ethyl, ⁇ -propyl, «-butyl, 2-methoxy ethyl, and 2-hydroxyethyl.
- R 4 is selected from the group consisting of hydrogen, alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl, wherein the alkyl, alkenyl, alkynyl, aryl, arylalkylenyl, aryloxyalkylenyl, alkylarylenyl, heteroaryl, heteroarylalkylenyl, heteroaryloxyalkylenyl, alkylheteroarylenyl, and heterocyclyl groups can be unsubstituted or substituted by one or more substituents independently selected from the group consisting of alkyl, alkoxy, hydroxyalkyl, haloalkyl, haloalkoxy
- R 9 is selected from the group consisting of hydrogen and alkyl. In certain embodiments, R 9 is alkyl. In certain embodiments, R 9 is hydrogen.
- Q is selected from the group consisting of -C(O)-, -C(O)-O-, -S(O) 2 -, and -C(Re)-N(R 8 )- .
- Q is a bond.
- V is selected from the group consisting Of-C(R 6 )-, -0-C(R 6 )-, -N(Rs)-C(R 6 )-, and -S(O) 2 -.
- V is -C(R 6 )-.
- V is -N(Rg)-C(Re)-.
- G 2 is selected from the group consisting of -X 2 -C(O)-R', ⁇ -aminoacyl, ⁇ -aminoacyl- ⁇ -aminoacyl, -X 2 -C(O)-O-R', -C(0)-N(R")R', and -S(O) 2 -R'.
- Y' is selected from the group consisting of hydrogen, Ci -6 alkyl, and benzyl.
- Y 0 is selected from the group consisting of Cj -6 alkyl, carboxy-Ci- 6 alkylenyl, amino-Cu alkylenyl, mono-N-Ci- 6 alky lamino-C I-4 alkylenyl, and di-N,N-Cu 6 alkylamino-Ci_ 4 alkylenyl.
- XIII, Gi is selected from the group consisting of -C(O)-R', ⁇ -aminoacyl, and -C(O)-O-R'.
- R' contains one to ten carbon atoms.
- ⁇ -aminoacyl is an ⁇ -C 2 - ⁇ aminoacyl group derived from an ⁇ -amino acid selected from the group consisting of racemic, D-, and L-amino acids containing a total of at least 2 carbon atoms and a total of up to 11 carbon atoms, and may also include one or more heteroatoms selected from the group consisting of O, S, and N.
- the present invention provides a method of inducing cytokine biosynthesis in an animal comprising administering an effective amount of a compound or salt of any one of Formulas I 5 H 5 III, IV, V, VI, VII, VIII, IX, X, XI 5 XII 5 XIII, XIV, or any one of the above embodiments or administering any one of the above pharmaceutical compositions to the animal.
- the cytokine is selected from the group consisting of IFN- ⁇ , TNF- ⁇ , IL-6, IL-IO, and IL- 12.
- the cytokine is IFN- ⁇ or TNF- ⁇ .
- the present invention provides a method of treating a viral disease in an animal comprising administering a therapeutically effective amount of a compound or salt of any one of Formulas I 5 H 5 III, IV 5 V 5 VI 5 VII, VIII, IX, X, XI, XII, XIII 5 XIV 5 or any one of the above embodiments or administering any one of the above pharmaceutical compositions to the animal.
- compounds of the invention can be prepared according to Reaction Scheme II, where R, R 2 , Z, n and m are as defined above.
- compounds of the invention can also be prepared according to Reaction Scheme III, where Rj, R 2 , X, Z, and m are as defined above, and RA 2 and RB 2 taken together form a fused benzene ring or fused pyridine ring wherein the benzene ring or pyridine ring is unsubstituted or substituted by one or more R groups.
- a ketone of Formula XXX is condensed with diethyl oxalate under Claisen condensation conditions to provide a ketoester of Formula XXXI.
- the reaction can be carried out by adding sodium f ⁇ rt-butoxide to a solution of diethyl oxalate and the ketone of Formula XXX in ethanol at ambient temperature.
- a ketoester of Formula XXXI reacts with a hydrazine of Formula R 2 NHNH 2 to provide a pyrazole carboxylate of Formula XXXII.
- the reaction is conveniently carried out by slowly adding the hydrazine to a solution of a compound of Formula XXXI in a suitable solvent such as acetic acid. The reaction can be carried out at ambient temperature.
- step (3) of Reaction Scheme III the ester group of a pyrazole carboxylate of Formula XXXII is converted to an amide.
- the amination can be carried out by adding ammonium hydroxide to the pyrazole carboxylate of Formula XXXII in a suitable solvent such as methanol and heating at an elevated temperature such as 100 0 C.
- the reaction can be carried out in a pressure vessel.
- step (3) can be carried out by first hydrolyzing a pyrazole carboxylate of Formula XXXII to a carboxylic acid and then converting the carboxylic acid to an amide.
- the ester hydrolysis can be carried out under basic conditions by combining a pyrazole carboxylate of Formula XXXII with lithium hydroxide or sodium hydroxide in water and in a suitable solvent such as methanol or ethanol.
- the reaction can be carried out at ambient temperature, and the carboxylic acid product can be isolated using conventional methods.
- the conversion of the carboxylic acid to a pyrazole carboxamide of Formula XXXIII can be carried out under coupling conditions by adding 1-hydroxybenzotriazole and l-(3-dimethylaminopropyl)-3- ethylcarbodiimide hydrochloride to a solution of the carboxylic acid in a suitable solvent such as jV,7V-dimethylformamide (DMF) at ambient temperature and then adding concentrated ammonium hydroxide.
- a suitable solvent such as jV,7V-dimethylformamide (DMF)
- a pyrazole carboxamide of Formula XXXIII is dehydrated to a pyrazole carbonitrile of Formula XXXIV.
- Suitable dehydrating agents include thionyl chloride, trifluoroacetic anhydride, and phosphorous oxychloride.
- the reaction is conveniently carried out by treating the pyrazole carboxamide of Formula XXXIII with phosphorous oxychloride and heating the reaction at an elevated temperature such as 90 °C.
- a pyrazole carbonitrile of Formula XXXIV is brominated to provide a bromo-substituted pyrazole carbonitrile of Formula XXXV.
- the bromination is conveniently carried out by adding bromine to a solution of the pyrazole carbonitrile of Formula XXXIV and potassium acetate in acetic acid. The reaction can be carried out at ambient temperature.
- a Suzuki coupling reaction is conveniently carried out by heating a mixture of the bromo-substituted pyrazole of Formula XXX, dichlorobis(triphenylphosphine)palladiurn(II) and a boron reagent of Formula XXXVI 5 where M is -B(OH) 2 or -B(0-alkyl) 2 , in the presence of a base such as potassium carbonate.
- the reaction is carried out in a suitable solvent such as 1 ,2-dimethoxyethane and can be heated at an elevated temperature such as 75 - 95 0 C.
- step (7) of Reaction Scheme III the amine and nitrile functionalities of a pyrazole of Formula XXXVII react under acidic conditions to form a pyrazolo [3 ,4- c]quinoline or pyrazolo [3 ,4-c]naphthyridine of Formula XXXVIII.
- the intramolecular addition is conveniently carried out by stirring acetyl chloride in ethanol and adding the resulting acidic solution to the pyrazole of Formula XXXVIII.
- the reaction is then heated at reflux to provide the pyrazolo [3 ,4-c]quinoline or pyrazolo[3,4-c]naphthyridine of Formula XXXVIII.
- Reaction Scheme IV where R], R 2 , X, Z, and m are as defined above and RA I and RB I are as defined below.
- step (1) of Reaction Scheme IV a bromo-substituted pyrazole carbonitrile of Formula XXXV undergoes a Sonogashira coupling reaction with (trimethylsilyl)acetylene to provide a pyrazole carbonitrile of Formula XXXIX.
- the reaction can be carried out according to the literature procedure, Sonogashira, K.; Tohda, Y.; Hagihara, N., Tetrahedron Lett, 4467 (1975).
- the iodo analog may be used as a starting material for Reaction Scheme IV.
- the iodo analog can be prepared from a pyrazole carbonitrile of Formula XXIV 5 shown in Reaction Scheme III.
- the iodination can be carried out by treating a pyrazole carbonitrile of Formula XXXIV with iodine monochloride in a suitable solvent such as dichloromethane in the presence of a base such as potassium carbonate.
- the reaction can be carried out at ambient temperature.
- step (2) of Reaction Scheme IV the trimethylsilyl group of the pyrazole of Formula XXXIX is removed to provide the pyrazole of Formula XL.
- Potassium carbonate in methanol or tetrabutylammonium fluoride in tetrahydrofuran can be used to carry out the transformation.
- step (3) of Reaction Scheme IV the acetylene of the pyrazole of Formula XL is alkylated using conventional synthetic methods, Jacobs, T. L. in Organic Reactions, 5, 1, (1949), to provide a pyrazole of Formula XLI.
- the reaction can be carried out by deprotonation of the compound of Formula XL with a base and reaction of the resulting carbanion with an electrophile of Formula Rei-Halide, for example, iodomethane.
- Step (3) can be omitted when RBI is hydrogen.
- steps (1) through (3) of Reaction Scheme IV may be replaced with one step from the iodo analog using a Sonogashira coupling reaction.
- the coupling can be carried out by combining an alkyne of Formula RBI -G ⁇ C-H, copper(I) iodide, dichlorobis(triphenyl ⁇ hosphine)palladium(II), and triethylamine in a suitable solvent such as acetom ' trile and then heating at an elevated temperature, such as the reflux temperature of the solvent.
- step (6) of Reaction Scheme IV a bromo-substituted pyrazolo[3,4-c]pyridin-4- amine of Formula XLIII undergoes a transition metal catalyzed coupling reaction with a reagent of Formula RAI-M, where R A I is alkenyl, alkoxy, and -N(R ⁇ 2 to provide a pyrazolo[3,4-c]pyridine-4-amine of Formula XLIV.
- step (6) can be carried out by coupling a compound of Formula XLIII with an alkyne under Sonogashira conditions as described in step (1) of this reaction scheme.
- the resulting alkyne can be reduced under conventional hydrogenation conditions to provide a compound of Formula XLIV, where RA 1 is alkenyl or alkyl.
- Step (6) may also be carried out by (i) protecting the amino group of the compound of Formula XLIII, for example, with a Boc group; (ii) performing a lithium-halogen exchange; (iii) treating with an electrophile of the Formula R A i-Halide, for example iodomethane; and (iv) deprotecting the amine to provide a compound of Formula XLIV.
- compounds of the invention can be prepared according to Reaction Scheme V, where Rj b and R 2! , are subsets of Rj and R 2 as defined above that do not include those substituents which would be susceptible to reduction under the acidic hydrogenation conditions of the reaction and R, X, Z, and n are as defined above.
- Reaction Scheme V a pyrazolo[3,4-c]quinoline of Formula XLV is reduced to provide a 6,7,8,9-tetrahydro-2i/-pyrazolo[3,4-c]quinoline of Formula XLVI, which is a subgenus of Formula IV.
- the reaction may be carried out under heterogeneous hydrogenation conditions by adding platinum (IV) oxide to a solution or suspension of a pyrazolo[3,4-c]quinoline of Formula XLV in a suitable solvent such as trifluoroacetic acid and placing the reaction under hydrogen pressure.
- the reduction may be carried out at an earlier stage in the synthetic pathway.
- Pyrazolo[3,4 ⁇ c]naphthyridines of the invention can be prepared by using an azaindole as the starting material in Reaction Scheme I. Azaindoles are known compounds. Some are commercially available and others can be prepared using known synthetic methods. Alternatively, pyrazolo[3,4-c]naphthyridines of the invention can be prepared by using an aminopyridine boronic acid in Reaction Scheme III. Aminopyridine boronic acids can be prepared using known methods, for example, by directed ortho metalation of Boc-protected aminopyridines and subsequent electr ⁇ philic substitution. Alternatively, for some isomers, halogen-lithium exchange and subsequent electrophilic substitution can be used.
- halogen-lithium exchange can be carried out on a 2-bromopyridine that has a protected amino group in the 3 -position; subsequent electrophilic substitution with tributyltin chloride and deprotection of the amino group provides 3 -amino-2-tri-n-butylstannylpyridme.
- 6 7,8,9-Tetrahydro-2H-pyrazolo[3,4-c]naphthyridines can be prepared by reducing pyrazolo[3,4-c]naphthyridines using the method of Reaction Scheme V.
- compounds can be further elaborated using conventional synthetic methods.
- a compound of Formula XLVII 5 can undergo acid mediated cleavage of the Boc group in step (1) to give a secondary amine that can be functionalized in step (2) with an acid chloride of Formula R 4 C(O)Cl 5 an acid anhydride of Formula (R 4 C(O)) I O, an alkyl chloroformate of Formula R 4 OC(O)Cl 5 a sulfonyl chloride of Formula R 4 S(O) 2 Cl, a sulfonic anydride of Formula (R 4 S(O) 2 ⁇ O 5 an isocyanate of formula R 4 NCO, or an isothiocyanate of formula R 4 NCS to provide a compound of Formula XLIX where R 4 is defined as above and Q is -C(O)-, -C(O)O-, -S(O) 2 -, -C(O)NH-, or -C(S)NH-.
- a compound of Formula XLVIII in Reaction Scheme VI can undergo alkylation of the secondary amine.
- the compound of Formula XLVIII may be reacted with aldehydes, alkyl halides or triflates to provide a compound Formula L in which R 8 is defined as above.
- a compound of Formula XLVIII with aqueous formaldehyde and a reducing agent such as sodium cyanoborohydride in an appropriate solvent such as methanol yields a compound of Formula L, where R 8 is a methyl group.
- an olefin of Formula LII is derivatized using conventional methods.
- a compound of Formula LII where X is a bond and R 1 is hydrogen can be prepared by reducing the olefin using conventional heterogeneous hydrogenation conditions.
- a compound of Formula LII can be treated with pivalonitrile in the presence of titanium tetrachloride; the resulting nitrile-substituted compound can be converted by convention methods to a compound of Formula LIII where X is a bond and Ri is -C(O)-NH 2 .
- a compound of Formula LII can also be treated with ammonium hydroxide followed by di ⁇ tert-butyl dicarbonate to provide a compound of Formula LIII where X is a bond and Rj is -NHBoc, which can be deprotected and treated according to the methods of Reaction Scheme VI to provide a variety of other compounds.
- compounds of the invention can be prepared according to Reaction Scheme VIII, where RA 2 , RB 23 R2, and Z are as defined above.
- step (1) of Reaction Scheme VIII an olefin of Formula LII is condensed with diethyl oxalate to provide a ketoester of Formula LIV.
- the reaction can be carried out as described in step (1) of Reaction Scheme III.
- step (2) of Reaction Scheme VIII a ketoester of Formula LIV reacts with a hydrazine of Formula R 2 NHNH 2 to provide a pyrazole carboxylate of Formula LV.
- the reaction can be carried out as described in step (2) of Reaction Scheme III.
- step (3) of Reaction Scheme VIII a pyrazole carboxylate of Formula LV is converted to a pyrazole carboxamide of Formula LVL
- the reaction can be carried out as described in step (3) of Reaction Scheme III.
- step (4) of Reaction Scheme VIII the olefinic bond in a compound of Formula LVI is oxidized to provide an epoxide of Formula LVII.
- the reaction can be carried out by treating a suspension of a compound of Formula LVI in a suitable solvent such as chloroform with 3-chloroperoxybenzoic acid. The reaction can be carried out at ambient temperature.
- step (5) of Reaction Scheme VIII the epoxide ring in a compound of Formula LVII is cleaved to provide a hydroxy substituted pyrazole carboxamide of Formula LVIII.
- the reaction can be carried out by treating a solution of a compound of Formula LVII in a suitable solvent such as ethanol with palladium on carbon and ammonium formate. The reaction can be carried out at ambient temperature.
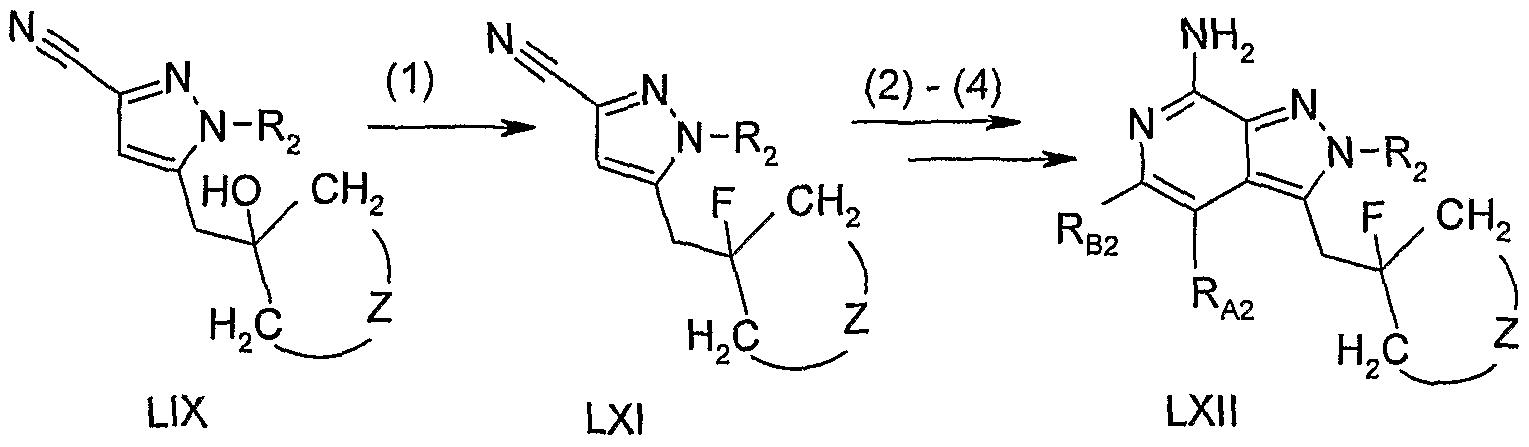
- a pyrazole carbonitrile of Formula LIX is converted to a pyrazolo[3,4 ⁇ c]quinoline or pyrazolo[3,4-c]naphthyridine of Formula LX.
- the conversion can be carried out using the methods described in steps (5) through (7) of Reaction Scheme III.
- compounds of the invention can be prepared according to Reaction Scheme IX, where R A2 , RB2, R 2 , and Z are as defined above.
- step (1) of Reaction Scheme IX 5 the hydroxy group in a pyrazolo carbonitrile of Formula LIX is replaced with a fluoro group to provide a pyrazolo carbonitrile of Formula LXI.
- the reaction can be carried out by treating a solution of a compound of Formula LIX in a suitable solvent such as dichloromethane with [bis(2-methoxyethyl)amino]sulfur trifiuoride.
- the trifluoride is added in a controlled fashion at a sub-ambient temperature such as 0 0 C.
- a pyrazole carbonitrile of Formula LXI is converted to a pyrazolo[3,4-c]quinoline or pyrazolo[3,4-c]naphthyridine of Formula LXII.
- the conversion can be carried out using the methods described in steps (5) through (7) of Reaction Scheme III.
- Compounds of the invention can also be prepared using variations of the routes shown in Reaction Schemes I through IX that would be apparent to one of skill in the art.
- a compound of Formula LII wherein Z is -N(Boc)- can be readily prepared from l-Boc-4-piperidinone according to the method of step (1) of Reaction Scheme VII.
- Steps (1) through (6) of Reaction Scheme VIII can then be used to prepare a compound of Formula LIX wherein Z is -N(Boc)-.
- Boc group can then be cleaved and the resulting amine can be treated with an acid chloride, alkyl chloroformate, sulfonyl chloride, sulfonic anhydride, isocyanate, or isothiocyanate according to the methods described in steps (1) and (2) of Reaction Scheme VI.
- Boc removal and amine functionalization steps can conveniently be carried out after the bromination in step (7) of Reaction Scheme VIII or after steps (1) and (2) of Reaction Scheme IX.
- compounds of the invention can be prepared according to Reaction Scheme X, where Ri, R 2 , RA 5 RB, G b Z, and m are as defined above.
- the amino group of a pyrazolo compound of Formula I can be converted by conventional methods to a functional group such as an amide, carbamate, urea, amidine, or another hydrolyzable group.
- reaction can be carried out, for example, by combining a compound of Formula I with a chloroformate or acid chloride, such as ethyl chloroformate or acetyl chloride, in the presence of a base such as triethylamine in a suitable solvent such as dichloromethane at room temperature.
- a chloroformate or acid chloride such as ethyl chloroformate or acetyl chloride
- compounds of the invention can be prepared according to Reaction Scheme XI, where R 2 , RA 5 RB, G 2 , Z, and m are as defined above.
- the hydrogen atom of the alcohol group of Formula LXIII can be replaced using conventional methods with a group such as Ci -6 alkanoyloxymethyl, 1-(Ci -6 alkanoyloxy)ethyl, 1 -methyl- 1-(C 1-6 alkanoyloxy)ethyl, Ci -6 alkoxycarbonyloxymethyl, iV-(C 1-6 alkoxycarbonyl)aminomethyl, succinoyl, Ci -6 alkanoyl, ⁇ -aminoCi- 4 alkanoyl, arylacyl, -P(O)(OH) 2 , -P(O)(O-C 1-6 alkyl) 2 , Ci -6 alkoxycarbonyl, C 1-6 alkylcarbamoyl, and ⁇ -aminoacyl or
- a therapeutically effective amount and “effective amount” mean an amount of the compound or salt sufficient to induce a therapeutic or prophylactic effect, such as cytokine induction, immunomodulation, antitumor activity, and/or antiviral activity.
- cytokine induction cytokine induction
- immunomodulation antitumor activity
- antiviral activity cytokine induction
- amount of compound or salt used in a pharmaceutical composition of the invention will vary according to factors known to those of skill in the art, such as the physical and chemical nature of the compound or salt, the nature of the carrier, and the intended dosing regimen.
- the method includes administering sufficient compound to provide a dose of from about 0.1 mg/m 2 to about 2.0 mg/ m 2 to the subject, for example, a dose of from about 0.4 mg/m 2 to about 1.2 mg/m 2 .
- dosage forms such as tablets, lozenges, capsules, parenteral formulations, syrups, creams, ointments, aerosol formulations, transdermal patches, transmucosal patches and the like.
- These dosage forms can be prepared with conventional pharmaceutically acceptable carriers and additives using conventional methods, which generally include the step of bringing the active ingredient into association with the carrier.
- the compounds or salts of the invention can be administered as the single therapeutic agent in the treatment regimen, or the compounds or salts described herein may be administered in combination with one another or with other active agents, including additional immune response modifiers, antivirals, antibiotics, antibodies, proteins, peptides, oligonucleotides, etc.
- Cytokines whose production may be induced by the administration of compounds or salts of the invention generally include interferon- ⁇ (IFN- ⁇ ) and tumor necrosis factor- ⁇ (TNF- ⁇ ) as well as certain interleukins (IL). Cytokines whose biosynthesis may be induced by compounds or salts of the invention include IFN- ⁇ , TNF- ⁇ , IL-I, IL-6, IL-IO and IL- 12, and a variety of other cytokines. Among other effects, these and other cytokines can inhibit virus production and tumor cell growth, making the compounds or salts useful in the treatment of viral diseases and neoplastic diseases. Accordingly, the invention provides a method of inducing cytokine biosynthesis in an animal comprising administering an effective amount of a compound or salt of the invention to the animal.
- compounds or salts described herein can affect other aspects of the innate immune response. For example, natural killer cell activity may be stimulated, an effect that may be due to cytokine induction.
- the compounds or salts may also activate macrophages, which in turn stimulate secretion of nitric oxide and the production of additional cytokines. Further, the compounds or salts may cause proliferation and differentiation of B-lymphocytes.
- T helper type 1 cytokine IFN- ⁇
- T helper type 2 cytokines IL- 4, IL-5 and IL- 13
- the compound or salt or composition may be administered alone or in combination with one or more active components as in, for example, a vaccine adjuvant.
- the compound or salt or composition and other component or components may be administered separately; together but independently such as in a solution; or together and associated with one another such as (a) covalently linked or (b) non-covalently associated, e.g., in a colloidal suspension.
- Conditions for which compounds or salts or compositions identified herein may be used as treatments include, but are not limited to:
- viral diseases such as, for example, diseases resulting from infection by an adenovirus, a herpesvirus (e.g., HSV-I, HSV-II, CMV, or VZV), a poxvirus (e.g., an orthopoxvirus such as variola or vaccinia, or molluscum contagiosum), a picornavirus (e.g., rhinovirus or enterovirus), an orthomyxovirus (e.g., influenzavirus), a paramyxovirus (e.g., parainfluenzavirus, mumps virus, measles virus, and respiratory syncytial virus (RSV)), a coronavirus (e.g., SARS), a papovavirus (e.g., papillomaviruses, such as those that cause genital warts, common warts, or plantar warts), a hepadnavirus (e.g., hepatitis B virus),
- atopic diseases such as atopic dermatitis or eczema, eosinophilia, asthma, allergy, allergic rhinitis, and Ommen's syndrome;
- autoimmune diseases such as systemic lupus erythematosus, essential thrombocythaemia, multiple sclerosis, discoid lupus, alopecia areata; and (g) diseases associated with wound repair such as, for example, inhibition of keloid formation and other types of scarring (e.g., enhancing wound healing, including chronic wounds).
- Compounds or salts identified herein may be particularly helpful in individuals having compromised immune function.
- compounds or salts may be used for treating the opportunistic infections and tumors that occur after suppression of cell mediated immunity in, for example, transplant patients, cancer patients and HIV patients.
- one or more of the above diseases or types of diseases for example, a viral disease or a neoplastic disease may be treated in an animal in need thereof (having the disease) by administering a therapeutically effective amount of a compound or salt of the invention to the animal.
- An animal may also be vaccinated by administering an effective amount of a compound or salt described herein, as a vaccine adjuvant.
- a method of vaccinating an animal comprising administering an effective amount of a compound or salt described herein to the animal as a vaccine adjuvant.
- the amount is expected to be a dose of, for example, from about 0.01 mg/m to about 5.0 mg/m , (computed according to the Dubois method as described above) although in some embodiments the induction or inhibition of cytokine biosynthesis may be performed by administering a compound or salt in a dose outside this range.
- the method includes administering sufficient compound or salt or composition to provide a dose of from about 0.1 mg/m 2 to about 2.0 mg/ m 2 to the subject, for example, a dose of from about 0.4 mg/m 2 to about 1.2 mg/m 2 .
- the invention also provides a method of treating a viral infection in an animal and a method of treating a neoplastic disease in an animal comprising administering an effective amount of a compound or salt of the invention to the animal.
- An amount effective to treat or inhibit a viral infection is an amount that will cause a reduction in one or more of the manifestations of viral infection, such as viral lesions, viral load, rate of virus production, and mortality as compared to untreated control animals.
- the precise amount that is effective for such treatment will vary according to factors known in the art but is expected to be a dose of about 100 ng/kg to about 50 mg/kg, preferably about 10 ⁇ g/kg to about 5 mg/kg.
- An amount of a compound or salt effective to treat a neoplastic condition is an amount that will cause a reduction in tumor size or in the number of tumor foci. Again, the precise amount will vary according to factors known in the art but is expected to be a dose of about 100 ng/kg to about 50 mg/kg, preferably about 10 ⁇ g/kg to about 5 mg/kg. In other embodiments, the amount is expected to be a dose of, for example, from about 0.01 mg/m 2 to about 5.0 mg/m 2 , (computed according to the Dubois method as described above) although in some embodiments either of these methods may be performed by administering a compound or salt in a dose outside this range.
- the method includes administering sufficient compound or salt to provide a dose of from about 0.1 mg/m 2 to about 2.0 mg/ m 2 to the subject, for example, a dose of from about 0.4 mg/m 2 to about 1.2 mg/m 2 .
- Tetrahydro-4/f-pyran-4-one (901 mg, 9.0 mmol) was added, and reaction mixture was stirred for 10 minutes.
- the -78 0 C bath was replaced with a 0 0 C bath, and then saturated aqueous ammonium chloride (30 mL) was added.
- the aqueous layer was separated and extracted three times with ter/-butyl methyl ether, and the combined organic fractions were dried over magnesium sulfate, filtered, and concentrated under reduced pressure.
- the residue (1.3 g) was purified by automated flash chromatography (eluting with ethyl acetate) and heated in refluxing 1 M hydrogen chloride in ethanol (50 mL) for one hour.
- the vessel was purged with nitrogen, and 10% palladium on carbon (400 mg) was added.
- the vessel was shaken under hydrogen pressure (50 psi, 3.4 X 10 5 Pa) for approximately ten minutes, and the reaction mixture was filtered through a layer of CELITE filter agent. The filter cake was washed with ethyl acetate. The filtrate was concentrated under reduced pressure, and the residue was dried under a stream of nitrogen to provide 3.80 g of l-tetrahydro-2H-pyran-4-ylacetone as a colorless oil.
- 2-Aminophenylboronic acid hydrochloride (1.39 g, 8.0 mmol) and dichlorobis(tri ⁇ henylphosphine)palladium(II) (140 mg, 0.20 mmol) were sequentially added to a mixture of 4-bromo-l-propyl-5-(tetrahydro-2H-pyran-4-ylmethyl)-lH- pyrazole-3-carbonitrile (1.25 g, 4.00 mmol), potassium carbonate (1.82 g, 13.2 mmol), 1,2-dimethoxyethane (DME) (15 mL), and water (7.5 mL). The flask was placed under vacuum and back-filled with nitrogen four times.
- dichlorobis(tri ⁇ henylphosphine)palladium(II) 140 mg, 0.20 mmol
- Part A tert-B ⁇ xXy ⁇ iV-(2-pyridyi)carbamate is available from the literature procedure (Moraczewski, A. L. et al, J. Org. Chem., 1998, 63, 7258) or can be prepared by the following method. Under a nitrogen atmosphere, sodium bis(trimethylsilyl)arnide (225 mL of a 1.0 M solution in tetrahydrofuran) was added over a period of 20 minutes to a solution of 2-aminopyridine (10.61 g, 108.0 mmol) in dry THF (150 mL). The solution was stirred for 15 minutes and then cooled to 0 0 C.
- Lithium hydroxide monohydrate (2.94 g, 70.2 mmol) was added to a solution of ethyl 1 -(2-methoxyethyl)-5-(tetrahydro-2i7 ⁇ pyran-4-ylmethyl)- 1 i/-pyrazole-3-carboxylate (5.2 g, 17.5 mmol) in methanol (60 mL) and water (20 niL). The mixture was stirred for overnight. Most of the volatiles were removed under reduced pressure, and acetic acid (40 mL) and water were added. The solution was cooled to approximately 0 0 C and stirred for one hour. The volatiles were removed under reduced pressure, and the residue was partitioned between water and chloroform.
- a solution of sodium tert-butoxide (5.29 g, 1.1 eq) in ethanol (51 mL) was added to a mixture of the material from Part A (LO eq) and diethyl oxalate (7.45 mL, 1.1 eq).
- the vessel containing the material from part A and diethyl oxalate was rinsed with additional ethanol (27 mL) and the rinse was added to the reaction mixture.
- the reaction mixture was stirred for 2 hours and then cooled to 0 °C.
- Acetic acid (57 mL) was added and the reaction mixture was stirred for 5 minutes.
- Methylhydrazine (2.64 mL, 1.0 eq) was added dropwise.
- Triethylamine (5.13 mL, 3.0 eq) was added to a stirred suspension of a portion of the material from Part F (2.91 g, 1.0 eq). The mixture was cooled to 0 °C and trifluoroacetic anhydride (5.14 mL, 3.0 eq) was added dropwise over a period of 5 minutes. The reaction mixture was stirred for 2 hours, quenched with saturated sodium carbonate (50 mL), and then allowed to warm to ambient temperature. Water (50 mL) and dichloromethane (200 mL) were added sequentially. The organic layer was separated, dried over magnesium sulfate, and then concentrated under reduced pressure to provide a yellow oil.
- the oil was dissolved in methanol (80 mL). Solid potassium carbonate (420 mg, 0.25 eq) was added and the mixture was stirred for 30 minutes. Aqueous hydrochloric acid (1.7 mL of 7 M, 1.0 eq) was added, the solution was stirred for 10 minutes, and then the bulk of the methanol was removed under reduced pressure. The residue was partitioned between dichloromethane (200 mL) and water (50 mL). The pH of the aqueous layer was adjusted to 7-8 with saturated sodium bicarbonate. The layers were separated and the aqueous layer was back extracted with dichloromethane (2 x 75 mL).
- Part H A solution of material from Part G (lots 1 and 2, 1.0 eq) in dichloromethane (41 mL) was cooled to 0 0 C. [Bis(2-methoxyethyl)amino]sulfur trifluoride (1.14 mL, 1.5 eq) was added dropwise. The reaction mixture was stirred for 45 minutes, quenched with saturated sodium bicarbonate (20 mL), and allowed to warm to ambient temperature. Saturated sodium bicarbonate (50 mL) and dichloromethane (50 mL) were added sequentially. The layers were separated and the aqueous layer was back extracted with dichloromethane (30 mL).
- the resulting foamy suspension was combined with dichloromethane (50 mL).
- the aqueous layer was adjusted to about pH 8 with 5% sodium hydroxide.
- the layers were separated and the aqueous layer was back extracted with dichloromethane (50 mL).
- the combined organics were dried over magnesium sulfate and then concentrated under reduced pressure to provide 0.59 g of a light yellow solid.
- This material was triturated with hot ethanol (about 8 mL), isolated by filtration, rinsed with ethanol (3 x 5 mL), and then dried (0.15 torr (20 Pa), 130 0 C, 2 hours) to provide 143 mg of l-[(4-fluorotetrahydro-2H ' -pyran-4-yl)methyl]-2-methyl-2H- ⁇ yrazolo[3,4-c]quinolin-4-amine as a tan powder, mp 252-254 0 C.
- the material from Part A (1.0 eq) was coupled with 2-aminophenylboronic acid hydrochloride (1.8 eq) and then cyclized using the general method of Example 7 Part J.
- the crude product was purified by automated flash chromatography (eluting with 20% CMA in chloroform for 3 column volumes, a gradient of 20-40% CMA in chloroform over 10 column volumes, and 40% CMA in chloroform for 3 column volumes) to provide a tan solid.
- This material was triturated with hot ethyl acetate (about 8 mL), isolated by filtration, rinsed with ethyl acetate (3 x 5 mL), and dried under high vacuum to provide 106 mg of 4-[(4-amino-2-methyl-2i/-pyrazolo[3,4-c]quinolin- 1 -yl)methyl]tetrahydro-2H- pyran-4-ol as a tan powder, mp 260-262 (dec) °C.
- This material was triturated with methanol (about 12 mL), isolated by filtration, rinsed with methanol (3 x 5 mL), and dried under high vacuum to provide 112 mg of 2-ethyl-l-[(4-fluorotetrahydro-2/J-pyran-4- yl)methyl]-2//-pyrazolo[3,4-c]quinolin-4-amine as a tan powder, mp 275-277 °C.
- Part A 4-[(4-Amino-2-ethyl-2/f-pyrazolo[3,4-c]quinolin-l -yl)methyl]piperidin-4-ol was prepared according to the method of Example 1 Part F using di(tert-butyl) 2-ethyl-l- methyl-2H-pyrazolo[3,4-c]quinolin-4-ylimidodicarbonate in lieu of di(tert-bntyl) 1- methyl-2 ⁇ propyl-2H-pyrazolo[3,4-c]quinolin-4-ylimidodicarbonate and tert-butyl A- oxopiperidine-1-carboxylate in lieu of cyclobutanone.
- Methanesulfonic anhydride (0.160 g, 0.922 mmol) was added to a slurry of 4-[(4- amino-2-ethyl-2H-pyrazolo[3,4-c]quinolin-l-yl)methyl]piperidin-4-ol (0.300 g, 0.922 mmol) in chloroform (10 mL). After 16 hours, 2M aqueous sodium carbonate was added and the biphasic mixture was stirred for 30 minutes resulting in a white precipitate. The mixture was extracted with 10% methanol in dichloromethane. The solution was concentrated.
- ⁇ -Propyl isocyanate (86 ⁇ L, 0.922 mmol) was added to a slurry of 4-[(4-amino-2- ethyl-2H-pyrazolo[3,4-c]quinolin-l-yl)methyl]piperidin-4-ol (0.300 g, 0.922 mmol) in chloroform (10 mL). After 16 hours, the solution was purified via automated flash chromatography eluting with a linear gradient of 2-25% CMA in chloroform.
- the acidic mixture was brought to pH 14 using 50% aqueous sodium hydroxide.
- the mixture was extracted with 10% methanol in dichloromethane, dried over anhydrous sodium sulfate, and concentrated.
- the material was purified via automated flash chromatography eluting with a linear gradient of 2-20% CMA in chloroform.
- the resulting white solid was triturated in acetonitrile and then isolated by filtration to provide 0.085 g of l-[(4-amino-2-ethyl- 6 5 7,8,9-tetrahydro-2H-pyrazolo[3,4-c]quinolin-l-yl)methyl]cyclohexanol as a white solid, m.p. 232-234 °C.
- MS (ESI) m/z 329.44 (M + H) +
- the material from Part C was treated according to the methods of Parts D, E, and F of Example 7.
- the crude solid obtained from Part F was partitioned between dichloromethane (200 mL) and water (100 mL).
- the aqueous solution was separated and extracted with chloroform (3 x 100 mL).
- the organic fractions were combined, dried over magnesium sulfate, and filtered.
- the filter cake was washed with chloroform (5 x 60 mL).
- the material from Part D was treated with triethylamine and trifluoroacetic anhydride according to the method of Part G of Example 7.
- the crude yellow oil that was obtained was purified by automated flash chromatography (eluting with a gradient of 30% hexanes in tert-butyl methyl ether to 100% tert-butyl methyl ether over 4 column volumes and then 100% tert-butyl methyl ether for 3 column volumes) and dried under high vacuum to provide 3.62 of the trifluoroacetate ester of tert-butyl 4-[(5-cyano-2-methyl- 27i-pyrazol-3-yl)methyl]-4-hydroxypiperidine-l -carboxylate as a colorless oil.
- Triethylamine (3.4 mL, 3.0 eq.) was added to a stirred suspension of 4-bromo-5- [(4-fluoropiperidin-4-yl)methyl]-l -methyl- lH-pyrazole-3-carbonitrile hydrochloride (2.7 g, 8.0 mmol) in dichloromethane (80 mL), and the mixture was cooled to 0 0 C.
- Acetyl chloride (0.74 mL, 1.3 eq.) was added, and the resulting solution was stirred for 1.5 hours and concentrated under reduced pressure.
- Triethylamine (1.5 mL, 1.5 eq.) was added to a stirred suspension of 4-bromo-5- [(4-fluoropiperidin-4-yl)methyl]-l -methyl- li?-pyrazole-3-carbonitrile hydrochloride (2.28 g, 6.75 mmol) in dichloromethane (67 mL), and isopropyl isocyanate (1.34 mL, 2.0 eq.) was added to the resulting solution. The reaction was stirred for 1.5 hours and concentrated under reduced pressure.
- Triethylamine (11.1 mL, 79.5 mmol) and methanesulfonyl chloride (1.24 mL, 15.9 mmol) were sequentially added to a mixture of the material from Part B in dichloromethane (80 mL), and the reaction was stirred for 5 minutes. Brine (40 mL) was added, and the aqueous layer was separated and extracted with dichloromethane (2 x 100 mL). The combined organic fractions were dried over magnesium sulfate, filtered, and concentrated under reduced pressure to provide a brown foam.
- the foam was purified by automated flash chromatography (eluting with a gradient of 0- 10% CMA in chloroform over 8 column volumes) to provide 1.9 g of 4-bromo-l-ethyl-5- ⁇ [4-fluoro-l- (methylsulfonyl)pi ⁇ eridin-4-yl]methyl ⁇ -lH-pyrazole-3-carbonitrile as a tan foam.
- Triethylamine (3.35 niL, 24.0 mmol) was added to a stirred suspension of 4- bromo-l-ethyl-5-[(4-fluoropiperidin-4-yl)methyl-lH-pyrazole-3-carbonitrile hydrochloride (2.52 g, 8.0 mmol) in dichloromethane (80 niL), and the resulting solution was cooled to 0 0 C.
- Acetyl chloride (0.74 mL, 10.4 mmol) was added dropwise, and the resulting solution was stirred for 1 hour. Water (50 mL) was added, and then the aqueous layer was separated and extracted with dichloromethane (2 x 100 mL).
- Triethylamine (2.23 mL, 16.0 mmol) and isopropyl isocyanate (1.01 niL, 10.4 mmol) were sequentially added to a mixture of 4-bromo-l-ethyl-5-[(4-fluoropiperidin-4- yl)methyl-l/7-pyrazole-3-carbonitrile hydrochloride (2.52 g, 8.0 mmol) and dichloromethane (40 mL). The reaction was stirred for 1.5 hours and diluted with dichloromethane (250 mL), washed with water (WO mL), dried over magnesium sulfate, filtered, and concentrated under reduced pressure to provide a brown foam.
- the foam was purified by automated flash chromatography (eluting with a gradient of 0-10% CMA in chloroform over 8 column volumes). The resulting off-white foam was concentrated from 1,2-dimethoxyethane (50 mL) and dried under high vacuum to provide 1.6 g of 4-[(4- bromo-5-cyano-2-ethyl-2i ⁇ -pyrazol-3-yl)methyl]-4-fluoro-N-isopropylpiperidine-l- carboxamide. Part B
- the foam was purified by automated flash chromatography (eluting with a gradient of 0-15% CMA in chloroform) to provide 1.5 g of 4-bromo-5- ⁇ [4-fluoro- 1 -(methylsulfonyl) ⁇ iperidin-4-yl]methyl ⁇ - 1 -propyl- lH-pyrazole- 3-carbonitrile as a white foam.
- the foam was concentrated from 1,2-dimethoxyethane (40 mL) before it was used in Part D. Part D
- the reaction conditions and purification methods described in Part J of Example 7 were used to treat the material from Part C.
- Isopropyl isocyanate (0.46 niL, 1.3 eq.) was added dropwise to a portion of the solution from Part B of Example 26 (21 mL) at room temperature. The resulting solution was stirred for 1 hour, diluted with dichloromethane (100 mL), washed with water (70 mL), dried over magnesium sulfate, filtered, and concentrated under reduced pressure to provide 1.48 g of a tan foam.
- the foam was purified by automated flash chromatography (eluting with a gradient of 0-10% CMA in chloroform over 8 column volumes) to provide 1.05 g of 4-(4-bromo-5-cyano-2-propyl-2#-pyrazol ⁇ 3-ylmemyl)-4-fluoro-iV- isopropylpiperidine- 1 -carboxamide as a white foam.
- Part B 4-(4-bromo-5-cyano-2-propyl-2#-pyrazol ⁇ 3-ylmemyl)-4-fluoro-iV- isopropylpiperidine- 1 -carboxamide as a white foam.
- Certain exemplary compounds including some of those described above in the Examples, have the following Formulas (IH-I 5 IV-I, V-I, or VIII-I) and the following R 2 , Z, Rj, and m substituents or variables, wherein each line of the table is matched with
- Certain exemplary compounds including some of those described above in the Examples, have the following Formulas (III-2. IV-2, V-2, and VIII-2) wherein R 2 , Q, R 4 , R 1 , and m are defined immediately below in the table. Each row of the table is matched . with Formula III-2, IV-2, V-2, or VIII-2 to represent a specific embodiment of the invention.
- Certain exemplary compounds including some of those described above in the Examples, have the following Formulas (III-4. IV-4, V-4, and VIII-4) wherein R 2 and m are defined immediately below in the table. Each row of the table is matched with Formula III-4, IV-4, V-4, or VIII-4 to represent a specific embodiment of the invention.
- Compounds of the invention have been found to modulate cytokine biosynthesis by inducing the production of interferon ⁇ and/or tumor necrosis factor ⁇ in human cells when tested using one of the methods described below.
- PBMC Peripheral blood mononuclear cells
- DPBS Dulbecco's Phosphate Buffered Saline
- HBSS Hank's Balanced Salts Solution
- whole blood is placed in Accuspin (Sigma) or LeucoSep (Greiner Bio-One, Inc., Longwood, FL) centrifuge frit tubes containing density gradient medium.
- Accuspin Sigma
- LeucoSep Garnier Bio-One, Inc., Longwood, FL centrifuge frit tubes containing density gradient medium.
- the PBMC layer is collected and washed twice with DPBS or HBSS and re-suspended at 4 x 10 6 cells/mL in RPMI complete.
- the PBMC suspension is added to 96 well flat bottom sterile tissue culture plates containing an equal volume of RPMI complete media containing test compound.
- the compounds are solubilized in dimethyl sulfoxide (DMSO).
- DMSO concentration should not exceed a final concentration of 1% for addition to the culture wells.
- the compounds are generally tested at concentrations ranging from 30-0.014 ⁇ M.
- Controls include cell samples with media only, cell samples with DMSO only (no compound), and cell samples with reference compound.
- test compound is added at 60 ⁇ M to the first well containing RPMI complete and serial 3 fold dilutions are made in the wells.
- the PBMC suspension is then added to the wells in an equal volume, bringing the test compound concentrations to the desired range (usually 30-0.014 ⁇ M).
- the final concentration of PBMC suspension is 2 x
- IFN- ⁇ concentration is determined with a human multi-subtype colorimetric sandwich ELISA (Catalog Number 41105) from PBL Biomedical Laboratories, Piscataway, NJ. Results are expressed in pg/mL.
- the data output of the assay consists of concentration values of TNF- ⁇ and
- IFN- ⁇ (y-axis) as a function of compound concentration (x-axis).
- the reference compound used is 2-[4-amino-2-ethoxymethyl-6,7,8,9-tetrahydro- ⁇ , ⁇ - dimethyl-lH-imidazo[4,5-c]qumolin-l-yl]ethanol hydrate (U.S. Patent No. 5,352,784; Example 91) and the expected area is the sum of the median dose values from the past 61 experiments.
- the minimum effective concentration is calculated based on the background- subtracted, reference-adjusted results for a given experiment and compound.
- the minimum effective concentration ( ⁇ molar) is the lowest of the tested compound concentrations that induces a response over a fixed cytokine concentration for the tested cytokine (usually 20 pg/mL for IFN- ⁇ and 40 pg/mL for TNF- ⁇ ).
- the maximal response is the maximal amount of cytokine (pg/ml) produced in the dose-response.
- PBMC Peripheral blood mononuclear cells
- the compounds are solubilized in dimethyl sulfoxide (DMSO).
- DMSO dimethyl sulfoxide
- Controls include cell samples with media only, cell samples with DMSO only (no compound), and cell samples with a reference compound 2-[4 ⁇ amino-2-ethoxymethyl-6,7,8,9-tetrahydro- ⁇ , ⁇ -dimethyl- lH-imidazo[4,5-c]quinolin-l-yl]ethanol hydrate (U.S. Patent No. 5,352,784; Example 91) on each plate.
- test compound is added at 7.5 mM to the first well of a dosing plate and serial 3 fold dilutions are made for the 7 subsequent concentrations in DMSO.
- RPMI Complete media is then added to the test compound dilutions in order to reach a final compound concentration of 2-fold higher (60 - 0.028 ⁇ M) than the final tested concentration range. Incubation
- test compound solution is then added to the wells containing the PBMC suspension bringing the test compound concentrations to the desired range (usually 30 ⁇ M - 0.014 ⁇ M) and the DMSO concentration to 0.4%.
- the final concentration of PBMC suspension is 2x10 6 cells/mL.
- the plates are covered with sterile plastic lids, mixed gently and then incubated for 18 to 24 hours at 37°C in a 5% carbon dioxide atmosphere.
- the data output of the assay consists of concentration values of TNF- ⁇ or
- IFN- ⁇ (y-axis) as a function of compound concentration (x-axis).
- a plate-wise scaling is performed within a given experiment aimed at reducing plate-to-plate variability associated within the same experiment.
- the greater of the median DMSO (DMSO control wells) or the experimental background (usually 20 pg/mL for IFN- ⁇ and 40 pg/mL for TNF- ⁇ ) is subtracted from each reading. Negative values that may result from background subtraction are set to zero.
- Each plate within a given experiment has a reference compound that serves as a control. This control is used to calculate a median expected area under the curve across all plates in the assay.
- a plate- wise scaling factor is calculated for each plate as a ratio of the area of the reference compound on the particular plate to the median expected area for the entire experiment.
- the data from each plate are then multiplied by the plate-wise scaling factor for all plates. Only data from plates bearing a scaling factor of between 0.5 and 2.0 (for both cytokines IFN- ⁇ , TNF- ⁇ ) are reported. Data from plates with scaling factors outside the above- mentioned interval are retested until they bear scaling factors inside the above mentioned interval. The above method produces a scaling of the y- values without altering the shape of the curve.
- the reference compound used is 2-[4-amino-2-ethoxymethyl-6,7,8,9- tetrahydro- ⁇ , ⁇ -dimethyl-lH-imidazo[4,5-c]quinolin-l-yl]ethanol hydrate (U.S. Patent No. 5,352,784; Example 91).
- the median expected area is the median area across all plates that are part of a given experiment.
- a second scaling may also be performed to reduce inter-experiment variability
- the adjustment ratio is the area of the reference compound in the new experiment divided by the expected area of the reference compound based on an average of previous experiments (unadjusted readings). This results in the scaling of the reading (y-axis) for the new data without changing the shape of the dose-response curve.
- the reference compound used is 2-[4- amino-2-ethoxymethyl-6,7,8,9-tetrahydro-a,a-dimethyl-lH-imidazo[4,5-c]quinolin-l- yljethanol hydrate (U.S. Patent No. 5,352,784; Example 91) and the expected area is the sum of the median dose values from an average of previous experiments.
- the minimum effective concentration is calculated based on the background- subtracted, reference-adjusted results for a given experiment and compound.
- the minimum effective concentration ( ⁇ molar) is the lowest of the tested compound concentrations that induces a response over a fixed cytokine concentration for the tested cytokine (usually 20 pg/mL for IFN- ⁇ and 40 pg/mL for TNF- ⁇ ).
- the maximal response is the maximal amount of cytokine (pg/ml) produced in the dose-response.
Abstract
Description
Claims
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP06749140A EP1863814A1 (en) | 2005-04-01 | 2006-03-31 | 1-substituted pyrazolo (3,4-c) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
| AU2006232375A AU2006232375A1 (en) | 2005-04-01 | 2006-03-31 | 1-substituted pyrazolo (3,4-c) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
| CA002602590A CA2602590A1 (en) | 2005-04-01 | 2006-03-31 | 1-substituted pyrazolo (3,4-c) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
| JP2008504494A JP2008538550A (en) | 2005-04-01 | 2006-03-31 | 1-Substituted pyrazolo (3,4-c) cyclic compounds as modulators of cytokine biosynthesis for treating viral infections and neoplastic diseases |
| US11/887,492 US7943636B2 (en) | 2005-04-01 | 2006-03-31 | 1-substituted pyrazolo (3,4-C) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US66786905P | 2005-04-01 | 2005-04-01 | |
| US60/667,869 | 2005-04-01 | ||
| US73303705P | 2005-11-03 | 2005-11-03 | |
| US60/733,037 | 2005-11-03 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2006107851A1 true WO2006107851A1 (en) | 2006-10-12 |
Family
ID=36763054
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2006/012263 WO2006107851A1 (en) | 2005-04-01 | 2006-03-31 | 1-substituted pyrazolo (3,4-c) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US7943636B2 (en) |
| EP (1) | EP1863814A1 (en) |
| JP (1) | JP2008538550A (en) |
| AU (1) | AU2006232375A1 (en) |
| CA (1) | CA2602590A1 (en) |
| WO (1) | WO2006107851A1 (en) |
Cited By (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7879849B2 (en) | 2003-10-03 | 2011-02-01 | 3M Innovative Properties Company | Pyrazolopyridines and analogs thereof |
| US7897597B2 (en) | 2003-08-27 | 2011-03-01 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted imidazoquinolines |
| US7897767B2 (en) | 2003-11-14 | 2011-03-01 | 3M Innovative Properties Company | Oxime substituted imidazoquinolines |
| US7906506B2 (en) | 2006-07-12 | 2011-03-15 | 3M Innovative Properties Company | Substituted chiral fused [1,2] imidazo [4,5-c] ring compounds and methods |
| US7915281B2 (en) | 2004-06-18 | 2011-03-29 | 3M Innovative Properties Company | Isoxazole, dihydroisoxazole, and oxadiazole substituted imidazo ring compounds and method |
| US7923429B2 (en) | 2003-09-05 | 2011-04-12 | 3M Innovative Properties Company | Treatment for CD5+ B cell lymphoma |
| US7943609B2 (en) | 2004-12-30 | 2011-05-17 | 3M Innovative Proprerties Company | Chiral fused [1,2]imidazo[4,5-C] ring compounds |
| US7943636B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | 1-substituted pyrazolo (3,4-C) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
| US7943610B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | Pyrazolopyridine-1,4-diamines and analogs thereof |
| US7968563B2 (en) | 2005-02-11 | 2011-06-28 | 3M Innovative Properties Company | Oxime and hydroxylamine substituted imidazo[4,5-c] ring compounds and methods |
| US8034938B2 (en) | 2004-12-30 | 2011-10-11 | 3M Innovative Properties Company | Substituted chiral fused [1,2]imidazo[4,5-c] ring compounds |
| US8598192B2 (en) | 2003-11-14 | 2013-12-03 | 3M Innovative Properties Company | Hydroxylamine substituted imidazoquinolines |
| US8673932B2 (en) | 2003-08-12 | 2014-03-18 | 3M Innovative Properties Company | Oxime substituted imidazo-containing compounds |
| US8691837B2 (en) | 2003-11-25 | 2014-04-08 | 3M Innovative Properties Company | Substituted imidazo ring systems and methods |
| US8735421B2 (en) | 2003-12-30 | 2014-05-27 | 3M Innovative Properties Company | Imidazoquinolinyl sulfonamides |
| US8802853B2 (en) | 2003-12-29 | 2014-08-12 | 3M Innovative Properties Company | Arylalkenyl and arylalkynyl substituted imidazoquinolines |
| US8871782B2 (en) | 2003-10-03 | 2014-10-28 | 3M Innovative Properties Company | Alkoxy substituted imidazoquinolines |
| US8951528B2 (en) | 2006-02-22 | 2015-02-10 | 3M Innovative Properties Company | Immune response modifier conjugates |
| US9248127B2 (en) | 2005-02-04 | 2016-02-02 | 3M Innovative Properties Company | Aqueous gel formulations containing immune response modifiers |
| TWI557121B (en) * | 2011-04-21 | 2016-11-11 | 原真股份有限公司 | Novel kinase inhibitors |
| EP3153180A1 (en) | 2011-06-03 | 2017-04-12 | 3M Innovative Properties Company | Heterobifunctional linkers with polyethylene glycol segments and immune response modifier conjugates made therefrom |
| US10000482B2 (en) | 2012-10-19 | 2018-06-19 | Origenis Gmbh | Kinase inhibitors |
| US10005772B2 (en) | 2006-12-22 | 2018-06-26 | 3M Innovative Properties Company | Immune response modifier compositions and methods |
Families Citing this family (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040265351A1 (en) | 2003-04-10 | 2004-12-30 | Miller Richard L. | Methods and compositions for enhancing immune response |
| SG149829A1 (en) | 2003-10-03 | 2009-02-27 | 3M Innovative Properties Co | Pyrazolopyridines and analogs thereof |
| CA2559863A1 (en) | 2004-03-24 | 2005-10-13 | 3M Innovative Properties Company | Amide substituted imidazopyridines, imidazoquinolines, and imidazonaphthyridines |
| US8017779B2 (en) | 2004-06-15 | 2011-09-13 | 3M Innovative Properties Company | Nitrogen containing heterocyclyl substituted imidazoquinolines and imidazonaphthyridines |
| US8541438B2 (en) | 2004-06-18 | 2013-09-24 | 3M Innovative Properties Company | Substituted imidazoquinolines, imidazopyridines, and imidazonaphthyridines |
| US8026366B2 (en) | 2004-06-18 | 2011-09-27 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted thiazoloquinolines and thiazolonaphthyridines |
| US7897609B2 (en) | 2004-06-18 | 2011-03-01 | 3M Innovative Properties Company | Aryl substituted imidazonaphthyridines |
| WO2007120121A2 (en) | 2005-02-09 | 2007-10-25 | Coley Pharmaceutical Group, Inc. | Oxime and hydroxylamine substituted thiazolo[4,5-c] ring compounds and methods |
| AU2006212765B2 (en) | 2005-02-09 | 2012-02-02 | 3M Innovative Properties Company | Alkyloxy substituted thiazoloquinolines and thiazolonaphthyridines |
| JP2008532933A (en) | 2005-02-11 | 2008-08-21 | コーリー ファーマシューティカル グループ,インコーポレイテッド | Substituted imidazoquinolines and substituted imidazonaphthyridines |
| JP2008543725A (en) | 2005-02-23 | 2008-12-04 | コーリー ファーマシューティカル グループ,インコーポレイテッド | Hydroxyalkyl substituted imidazoquinolines |
| AU2006216686A1 (en) | 2005-02-23 | 2006-08-31 | Coley Pharmaceutical Group, Inc. | Method of preferentially inducing the biosynthesis of interferon |
| WO2006091567A2 (en) | 2005-02-23 | 2006-08-31 | Coley Pharmaceutical Group, Inc. | Hydroxyalkyl substituted imidazoquinoline compounds and methods |
| ZA200803029B (en) | 2005-09-09 | 2009-02-25 | Coley Pharm Group Inc | Amide and carbamate derivatives of alkyl substituted /V-[4-(4-amino-1H-imidazo[4,5-c] quinolin-1-yl)butyl] methane-sulfonamides and methods |
| US8476292B2 (en) | 2005-09-09 | 2013-07-02 | 3M Innovative Properties Company | Amide and carbamate derivatives of N-{2-[4-amino-2-(ethoxymethyl)-1H-imidazo[4,5-c] quinolin-1-Yl]-1,1-dimethylethyl}methanesulfonamide and methods |
| EP1948173B1 (en) | 2005-11-04 | 2013-07-17 | 3M Innovative Properties Company | Hydroxy and alkoxy substituted 1h-imidazoquinolines and methods |
| WO2007106854A2 (en) | 2006-03-15 | 2007-09-20 | Coley Pharmaceutical Group, Inc. | Hydroxy and alkoxy substituted 1h-imidazonaphthyridines and methods |
| US8178539B2 (en) | 2006-09-06 | 2012-05-15 | 3M Innovative Properties Company | Substituted 3,4,6,7-tetrahydro-5H-1,2a,4a,8-tetraazacyclopenta[cd]phenalenes and methods |
| DK2268618T3 (en) * | 2008-03-03 | 2015-08-17 | Novartis Ag | Compounds and compositions as TLR aktivitetsmodulatorer |
| MX2011010050A (en) | 2009-03-25 | 2011-12-14 | Univ Texas | Compositions for stimulation of mammalian innate immune resistance to pathogens. |
| DK2606047T3 (en) | 2010-08-17 | 2017-03-27 | 3M Innovative Properties Co | COMPOSITIONS AND FORMULATIONS WITH LIPIDIZED IMMUNE RESPONSE-MODIFIING COMPOUND AND PROCEDURES THEREOF |
| CN103582496B (en) | 2011-06-03 | 2016-05-11 | 3M创新有限公司 | There is the Heterobifunctional connection base of polyethylene glycol segment and the immune response modifier conjugate of being made by it |
| WO2016044839A2 (en) | 2014-09-19 | 2016-03-24 | The Board Of Regents Of The University Of Texas System | Compositions and methods for treating viral infections through stimulated innate immunity in combination with antiviral compounds |
| JP6137225B2 (en) * | 2015-03-04 | 2017-05-31 | トヨタ自動車株式会社 | Bumper absorber mounting structure |
| GB201617758D0 (en) * | 2016-10-20 | 2016-12-07 | Almac Discovery Limited | Pharmaceutical compounds |
| CA3086439A1 (en) | 2017-12-20 | 2019-06-27 | 3M Innovative Properties Company | Amide substitued imidazo[4,5-c]quinoline compounds with a branched chain linking group for use as an immune response modifier |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4689338A (en) * | 1983-11-18 | 1987-08-25 | Riker Laboratories, Inc. | 1H-Imidazo[4,5-c]quinolin-4-amines and antiviral use |
| WO1995002598A1 (en) * | 1993-07-15 | 1995-01-26 | Minnesota Mining And Manufacturing Company | Fused cycloalkylimidazopyridines |
| EP1104764A1 (en) * | 1998-08-12 | 2001-06-06 | Hokuriku Seiyaku Co., Ltd. | 1h-imidazopyridine derivatives |
| WO2004058759A1 (en) * | 2002-12-20 | 2004-07-15 | 3M Innovative Properties Company | Aryl / hetaryl substituted imidazoquinolines |
| WO2005079195A2 (en) * | 2003-10-03 | 2005-09-01 | 3M Innovative Properties Company | Pyrazolopyridines and analogs thereof |
| WO2006028545A2 (en) * | 2004-06-18 | 2006-03-16 | 3M Innovative Properties Company | Substituted imidazoquinolines, imidazopyridines, and imidazonaphthyridines |
Family Cites Families (310)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3314941A (en) | 1964-06-23 | 1967-04-18 | American Cyanamid Co | Novel substituted pyridodiazepins |
| DE1645976A1 (en) | 1966-06-18 | 1971-01-21 | Ajinomoto Kk | Process for the preparation of adenosine and 2 ', 3'-O-isopropylidene adenosine |
| ZA704419B (en) | 1969-07-21 | 1971-04-28 | Ici Australia Ltd | Injectable aqueous solutions of tetramisole |
| US3692907A (en) | 1970-10-27 | 1972-09-19 | Richardson Merrell Inc | Treating viral infections with bis-basic ethers and thioethers of fluorenone and fluorene and pharmaceutical compositons of the same |
| US3917624A (en) | 1972-09-27 | 1975-11-04 | Pfizer | Process for producing 2-amino-nicotinonitrile intermediates |
| US4006237A (en) | 1973-10-11 | 1977-02-01 | Beecham Group Limited | Tetrahydrocarbostyril derivatives for the prophylaxis of asthma, hayfever and rhinitis |
| US3891660A (en) | 1974-02-07 | 1975-06-24 | Squibb & Sons Inc | Derivatives of 1H-imidazo{8 4,5-c{9 pyridine-7-carboxylic acids and esters |
| US3899508A (en) | 1974-04-12 | 1975-08-12 | Lilly Co Eli | 5-(2-Aminophenyl)pyrazole-3-carboxylic acids and esters thereof |
| DE2423389A1 (en) | 1974-05-14 | 1975-12-04 | Hoechst Ag | PSYCHOTROPIC MEDICINAL PRODUCTS AND THE METHOD OF MANUFACTURING THEREOF |
| JPS6016438B2 (en) | 1976-10-14 | 1985-04-25 | ウェルファイド株式会社 | Imidazoquinoline derivatives |
| US4381344A (en) | 1980-04-25 | 1983-04-26 | Burroughs Wellcome Co. | Process for producing deoxyribosides using bacterial phosphorylase |
| DE3204126A1 (en) | 1982-02-06 | 1983-08-11 | Bayer Ag, 5090 Leverkusen | PYRAZOLOXAZINE, -THIAZINE, -CHINOLINE, METHOD FOR THE PRODUCTION THEREOF AND THEIR USE AS A MEDICINAL PRODUCT |
| US4758574A (en) | 1982-05-03 | 1988-07-19 | Eli Lilly And Company | 2-phenylimidazio (4,5-c) pyridines |
| US4563525A (en) | 1983-05-31 | 1986-01-07 | Ici Americas Inc. | Process for preparing pyrazolopyridine compounds |
| CA1271477A (en) | 1983-11-18 | 1990-07-10 | John F. Gerster | 1h-imidazo[4,5-c]quinolin-4-amines |
| ZA848968B (en) | 1983-11-18 | 1986-06-25 | Riker Laboratories Inc | 1h-imidazo(4,5-c)quinolines and 1h-imidazo(4,5-c)quinolin-4-amines |
| JPS61112075A (en) | 1984-11-05 | 1986-05-30 | Shionogi & Co Ltd | Thienylpyrazoloquinoline derivative |
| US4668686A (en) | 1985-04-25 | 1987-05-26 | Bristol-Myers Company | Imidazoquinoline antithrombrogenic cardiotonic agents |
| US4593821A (en) | 1985-04-25 | 1986-06-10 | Laros Equipment Company, Inc. | Belt separator for blow molding parts |
| US4698346A (en) | 1985-05-08 | 1987-10-06 | Usv Pharmaceutical Corporation | Thiazolo[5,4-h]quinoline compounds useful as anti-allergy agents |
| US4826830A (en) | 1985-07-31 | 1989-05-02 | Jui Han | Topical application of glyciphosphoramide |
| CA1306260C (en) | 1985-10-18 | 1992-08-11 | Shionogi & Co., Ltd. | Condensed imidazopyridine derivatives |
| HU197019B (en) | 1985-11-12 | 1989-02-28 | Egyt Gyogyszervegyeszeti Gyar | Process for producing thiqzolo (4,5-c) quinoline derivatives and pharmaceuticals comprising same |
| US4837378A (en) | 1986-01-15 | 1989-06-06 | Curatek Pharmaceuticals, Inc. | Topical metronidazole formulations and therapeutic uses thereof |
| JPS6310787A (en) | 1986-03-06 | 1988-01-18 | Takeda Chem Ind Ltd | Nucleotide analog, production thereof and antiviral agent |
| US4775674A (en) | 1986-05-23 | 1988-10-04 | Bristol-Myers Company | Imidazoquinolinylether derivatives useful as phosphodiesterase and blood aggregation inhibitors |
| CA1287061C (en) | 1986-06-27 | 1991-07-30 | Roche Holding Ltd. | Pyridine ethanolamine derivatives |
| US5500228A (en) | 1987-05-26 | 1996-03-19 | American Cyanamid Company | Phase separation-microencapsulated pharmaceuticals compositions useful for alleviating dental disease |
| US4880779A (en) | 1987-07-31 | 1989-11-14 | Research Corporation Technologies, Inc. | Method of prevention or treatment of AIDS by inhibition of human immunodeficiency virus |
| US4774339A (en) | 1987-08-10 | 1988-09-27 | Molecular Probes, Inc. | Chemically reactive dipyrrometheneboron difluoride dyes |
| JPH01180156A (en) | 1988-01-12 | 1989-07-18 | Nec Corp | Packet switching circuit |
| US5536743A (en) | 1988-01-15 | 1996-07-16 | Curatek Pharmaceuticals Limited Partnership | Intravaginal treatment of vaginal infections with buffered metronidazole compositions |
| US5225183A (en) | 1988-12-06 | 1993-07-06 | Riker Laboratories, Inc. | Medicinal aerosol formulations |
| US5736553A (en) | 1988-12-15 | 1998-04-07 | Riker Laboratories, Inc. | Topical formulations and transdermal delivery systems containing 1-isobutyl-1H-imidazo 4,5-C!quinolin-4-amine |
| US5238944A (en) | 1988-12-15 | 1993-08-24 | Riker Laboratories, Inc. | Topical formulations and transdermal delivery systems containing 1-isobutyl-1H-imidazo[4,5-c]quinolin-4-amine |
| US5756747A (en) | 1989-02-27 | 1998-05-26 | Riker Laboratories, Inc. | 1H-imidazo 4,5-c!quinolin-4-amines |
| DE69029212T2 (en) | 1989-02-27 | 1997-05-22 | Riker Laboratories Inc | 4-Amino-1H-imidazo (4,5-c) quinolines as antiviral agents |
| US5457183A (en) | 1989-03-06 | 1995-10-10 | Board Of Regents, The University Of Texas System | Hydroxylated texaphyrins |
| US4929624A (en) | 1989-03-23 | 1990-05-29 | Minnesota Mining And Manufacturing Company | Olefinic 1H-imidazo(4,5-c)quinolin-4-amines |
| US5037986A (en) | 1989-03-23 | 1991-08-06 | Minnesota Mining And Manufacturing Company | Olefinic 1H-imidazo[4,5-c]quinolin-4-amines |
| NZ232740A (en) | 1989-04-20 | 1992-06-25 | Riker Laboratories Inc | Solution for parenteral administration comprising a 1h-imidazo(4,5-c) quinolin-4-amine derivative, an acid and a tonicity adjuster |
| US4988815A (en) | 1989-10-26 | 1991-01-29 | Riker Laboratories, Inc. | 3-Amino or 3-nitro quinoline compounds which are intermediates in preparing 1H-imidazo[4,5-c]quinolines |
| EP0452331A1 (en) | 1989-11-02 | 1991-10-23 | PFEIFER & LANGEN | Process and device for preventing crust formation in continuously operating sugar-crystallization equipment |
| US5750134A (en) | 1989-11-03 | 1998-05-12 | Riker Laboratories, Inc. | Bioadhesive composition and patch |
| JPH05503517A (en) | 1989-12-18 | 1993-06-10 | バージニア・コモンウェルス・ユニバーシティ | Sigma receptor ligand and its uses |
| US5274113A (en) | 1991-11-01 | 1993-12-28 | Molecular Probes, Inc. | Long wavelength chemically reactive dipyrrometheneboron difluoride dyes and conjugates |
| US5530114A (en) | 1990-04-30 | 1996-06-25 | Isis Pharmaceuticals, Inc. | Oligonucleotide modulation of arachidonic acid metabolism |
| AU653524B2 (en) | 1990-06-08 | 1994-10-06 | Roussel-Uclaf | New imidazole derivatives, their preparation process, the new intermediates obtained, their use as medicaments and the pharmaceutical compositions containing them |
| JP2660086B2 (en) | 1990-07-03 | 1997-10-08 | 明治製菓株式会社 | Agent for improving brain and cardiac dysfunction |
| WO1992006093A1 (en) | 1990-10-05 | 1992-04-16 | Minnesota Mining And Manufacturing Company | Process for the preparation of imidazo[4,5-c]quinolin-4-amines |
| MX9203481A (en) | 1990-10-18 | 1992-07-01 | Minnesota Mining & Mfg | FORMULATIONS. |
| US5248782A (en) | 1990-12-18 | 1993-09-28 | Molecular Probes, Inc. | Long wavelength heteroaryl-substituted dipyrrometheneboron difluoride dyes |
| US5175296A (en) | 1991-03-01 | 1992-12-29 | Minnesota Mining And Manufacturing Company | Imidazo[4,5-c]quinolin-4-amines and processes for their preparation |
| HU222250B1 (en) | 1991-03-01 | 2003-05-28 | Minnesota Mining And Manufacturing Company | Intermediates for producing 1- and 2-substituted 1h-imidazo[4,5-c]quinolin-4-amine derivatives |
| US5389640A (en) | 1991-03-01 | 1995-02-14 | Minnesota Mining And Manufacturing Company | 1-substituted, 2-substituted 1H-imidazo[4,5-c]quinolin-4-amines |
| JPH04327587A (en) | 1991-04-26 | 1992-11-17 | Asahi Chem Ind Co Ltd | 6'-c-alkyl-3-deazaneplanocin a derivative, its production and use |
| US5187288A (en) | 1991-05-22 | 1993-02-16 | Molecular Probes, Inc. | Ethenyl-substituted dipyrrometheneboron difluoride dyes and their synthesis |
| US5268376A (en) | 1991-09-04 | 1993-12-07 | Minnesota Mining And Manufacturing Company | 1-substituted 1H-imidazo[4,5-c]quinolin-4-amines |
| TW300219B (en) | 1991-09-14 | 1997-03-11 | Hoechst Ag | |
| PH31245A (en) | 1991-10-30 | 1998-06-18 | Janssen Pharmaceutica Nv | 1,3-Dihydro-2H-imidazoÄ4,5-BÜ-quinolin-2-one derivatives. |
| US5266575A (en) | 1991-11-06 | 1993-11-30 | Minnesota Mining And Manufacturing Company | 2-ethyl 1H-imidazo[4,5-ciquinolin-4-amines |
| US5378848A (en) | 1992-02-12 | 1995-01-03 | Shionogi & Co., Ltd. | Condensed imidazopyridine derivatives |
| IL105325A (en) | 1992-04-16 | 1996-11-14 | Minnesota Mining & Mfg | Immunogen/vaccine adjuvant composition |
| EP0641192B1 (en) | 1992-05-18 | 1997-07-23 | Minnesota Mining And Manufacturing Company | Transmucosal drug delivery device |
| US5352680A (en) | 1992-07-15 | 1994-10-04 | Regents Of The University Of Minnesota | Delta opioid receptor antagonists to block opioid agonist tolerance and dependence |
| US6608201B2 (en) | 1992-08-28 | 2003-08-19 | 3M Innovative Properties Company | Process for preparing 1-substituted, 2-substituted 1H-imidazo[4,5-c]quinolin-4-amines |
| US5395937A (en) | 1993-01-29 | 1995-03-07 | Minnesota Mining And Manufacturing Company | Process for preparing quinoline amines |
| EP0689424B1 (en) | 1993-03-17 | 1998-10-14 | Minnesota Mining And Manufacturing Company | Aerosol formulation containing an ester-, amide-, or mercaptoester-derived dispersing aid |
| DE4309969A1 (en) | 1993-03-26 | 1994-09-29 | Bayer Ag | Substituted hetero-fused imidazoles |
| DE69314318T2 (en) | 1993-04-27 | 1998-04-09 | Agfa Gevaert Nv | Method for inserting a water-soluble compound into a hydrophilic layer |
| US5648516A (en) | 1994-07-20 | 1997-07-15 | Minnesota Mining And Manufacturing Company | Fused cycloalkylimidazopyridines |
| ES2149276T3 (en) | 1993-07-15 | 2000-11-01 | Minnesota Mining & Mfg | IMIDAZO (4,5-C) PIRIDIN-4-AMINAS. |
| CA2131680C (en) | 1993-09-17 | 2006-11-07 | Gerhard Stucky | Process for preparing imidazopyridine derivatives |
| US5837809A (en) | 1995-08-11 | 1998-11-17 | Oregon Health Sciences University | Mammalian opioid receptor ligand and uses |
| JPH07163368A (en) | 1993-12-15 | 1995-06-27 | Hayashibara Biochem Lab Inc | Recombinant dna and transformant containing the same recombinant dna |
| TW574214B (en) | 1994-06-08 | 2004-02-01 | Pfizer | Corticotropin releasing factor antagonists |
| US6207646B1 (en) | 1994-07-15 | 2001-03-27 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| US6239116B1 (en) | 1994-07-15 | 2001-05-29 | University Of Iowa Research Foundation | Immunostimulatory nucleic acid molecules |
| PT772619E (en) | 1994-07-15 | 2006-10-31 | Univ Iowa Res Found | OLIGONUCLEOTIDOS IMUNOMODULADORES |
| US5612377A (en) | 1994-08-04 | 1997-03-18 | Minnesota Mining And Manufacturing Company | Method of inhibiting leukotriene biosynthesis |
| US5644063A (en) | 1994-09-08 | 1997-07-01 | Minnesota Mining And Manufacturing Company | Imidazo[4,5-c]pyridin-4-amine intermediates |
| GB9420168D0 (en) | 1994-10-06 | 1994-11-23 | Boots Co Plc | Therapeutic agents |
| US5571819A (en) | 1994-11-22 | 1996-11-05 | Sabb; Annmarie L. | Imidazopyridines as muscarinic agents |
| US5482936A (en) | 1995-01-12 | 1996-01-09 | Minnesota Mining And Manufacturing Company | Imidazo[4,5-C]quinoline amines |
| US6071949A (en) | 1995-03-14 | 2000-06-06 | The United States Of America As Represented By The Department Of Health And Human Services | Use of lipoxygenase inhibitors as anti-cancer therapeutic and intervention agents |
| US5585612A (en) | 1995-03-20 | 1996-12-17 | Harp Enterprises, Inc. | Method and apparatus for voting |
| FR2732605B1 (en) | 1995-04-07 | 1997-05-16 | Pasteur Merieux Serums Vacc | COMPOSITION FOR INDUCING MUCOSAL IMMUNE RESPONSE |
| US5766789A (en) | 1995-09-29 | 1998-06-16 | Energetics Systems Corporation | Electrical energy devices |
| DE69628804T2 (en) | 1995-12-08 | 2003-12-18 | Pfizer | Substituted heterocyclic derivatives as CRF antagonists |
| JPH09208584A (en) | 1996-01-29 | 1997-08-12 | Terumo Corp | Amide derivative, pharmaceutical preparation containing the same, and intermediate for synthesizing the same |
| US5939047A (en) | 1996-04-16 | 1999-08-17 | Jernberg; Gary R. | Local delivery of chemotherapeutic agents for treatment of periodontal disease |
| US5861268A (en) | 1996-05-23 | 1999-01-19 | Biomide Investment Limited Partnership | Method for induction of tumor cell apoptosis with chemical inhibitors targeted to 12-lipoxygenase |
| US5741908A (en) | 1996-06-21 | 1998-04-21 | Minnesota Mining And Manufacturing Company | Process for reparing imidazoquinolinamines |
| US5693811A (en) | 1996-06-21 | 1997-12-02 | Minnesota Mining And Manufacturing Company | Process for preparing tetrahdroimidazoquinolinamines |
| WO1998001448A1 (en) | 1996-07-03 | 1998-01-15 | Japan Energy Corporation | Novel purine derivatives |
| US6387938B1 (en) | 1996-07-05 | 2002-05-14 | Mochida Pharmaceutical Co., Ltd. | Benzimidazole derivatives |
| KR100518903B1 (en) | 1996-10-25 | 2005-10-06 | 미네소타 마이닝 앤드 매뉴팩춰링 캄파니 | Immune response modifier compounds for treatment of the th2 mediated and related diseases |
| US5939090A (en) | 1996-12-03 | 1999-08-17 | 3M Innovative Properties Company | Gel formulations for topical drug delivery |
| US6069149A (en) | 1997-01-09 | 2000-05-30 | Terumo Kabushiki Kaisha | Amide derivatives and intermediates for the synthesis thereof |
| US6406705B1 (en) | 1997-03-10 | 2002-06-18 | University Of Iowa Research Foundation | Use of nucleic acids containing unmethylated CpG dinucleotide as an adjuvant |
| US6426334B1 (en) | 1997-04-30 | 2002-07-30 | Hybridon, Inc. | Oligonucleotide mediated specific cytokine induction and reduction of tumor growth in a mammal |
| US6303347B1 (en) | 1997-05-08 | 2001-10-16 | Corixa Corporation | Aminoalkyl glucosaminide phosphate compounds and their use as adjuvants and immunoeffectors |
| US6113918A (en) | 1997-05-08 | 2000-09-05 | Ribi Immunochem Research, Inc. | Aminoalkyl glucosamine phosphate compounds and their use as adjuvants and immunoeffectors |
| AU7690898A (en) | 1997-05-20 | 1998-12-11 | Ottawa Civic Hospital Loeb Research Institute | Vectors and methods for immunization or therapeutic protocols |
| US6123957A (en) | 1997-07-16 | 2000-09-26 | Jernberg; Gary R. | Delivery of agents and method for regeneration of periodontal tissues |
| KR100528112B1 (en) | 1997-08-20 | 2006-03-17 | 소니 가부시끼 가이샤 | Apparatus and method for manufacturing disc-shaped recording medium |
| US6309623B1 (en) | 1997-09-29 | 2001-10-30 | Inhale Therapeutic Systems, Inc. | Stabilized preparations for use in metered dose inhalers |
| DE69817393T2 (en) | 1997-11-28 | 2004-06-17 | Sumitomo Pharmaceuticals Co., Ltd. | NEW HETEROCYCLIC CONNECTIONS |
| US6121323A (en) | 1997-12-03 | 2000-09-19 | 3M Innovative Properties Company | Bishydroxyureas |
| UA67760C2 (en) | 1997-12-11 | 2004-07-15 | Міннесота Майнінг Енд Мануфакчурінг Компані | Imidazonaphthyridines and use thereof to induce the biosynthesis of cytokines |
| TW572758B (en) | 1997-12-22 | 2004-01-21 | Sumitomo Pharma | Type 2 helper T cell-selective immune response inhibitors comprising purine derivatives |
| JPH11222432A (en) | 1998-02-03 | 1999-08-17 | Terumo Corp | Preparation for external use containing amide derivative inducing interferon |
| US6114058A (en) | 1998-05-26 | 2000-09-05 | Siemens Westinghouse Power Corporation | Iron aluminide alloy container for solid oxide fuel cells |
| US6110929A (en) | 1998-07-28 | 2000-08-29 | 3M Innovative Properties Company | Oxazolo, thiazolo and selenazolo [4,5-c]-quinolin-4-amines and analogs thereof |
| US5962636A (en) | 1998-08-12 | 1999-10-05 | Amgen Canada Inc. | Peptides capable of modulating inflammatory heart disease |
| US6518280B2 (en) | 1998-12-11 | 2003-02-11 | 3M Innovative Properties Company | Imidazonaphthyridines |
| EP1495758A3 (en) | 1999-01-08 | 2005-04-13 | 3M Innovative Properties Company | Formulations and methods for treatment of mucosal associated conditions with an immune response modifier |
| AU776654B2 (en) | 1999-01-08 | 2004-09-16 | 3M Innovative Properties Company | Formulations and methods for treatment of mucosal associated conditions with an immune response modifier |
| US6486168B1 (en) | 1999-01-08 | 2002-11-26 | 3M Innovative Properties Company | Formulations and methods for treatment of mucosal associated conditions with an immune response modifier |
| US20020058674A1 (en) | 1999-01-08 | 2002-05-16 | Hedenstrom John C. | Systems and methods for treating a mucosal surface |
| US6558951B1 (en) | 1999-02-11 | 2003-05-06 | 3M Innovative Properties Company | Maturation of dendritic cells with immune response modifying compounds |
| US6294271B1 (en) | 1999-02-12 | 2001-09-25 | Shin-Etsu Chemical Co., Ltd. | Flip-chip type semiconductor device sealing material and flip-chip type semiconductor device |
| JP2000247884A (en) | 1999-03-01 | 2000-09-12 | Sumitomo Pharmaceut Co Ltd | Arachidonic acid-induced skin disease-treating agent |
| WO2000061765A2 (en) | 1999-04-12 | 2000-10-19 | Lexicon Genetics Incorporated | Lipoxygenase proteins and polynucleotides encoding the same |
| US6331539B1 (en) | 1999-06-10 | 2001-12-18 | 3M Innovative Properties Company | Sulfonamide and sulfamide substituted imidazoquinolines |
| US6451810B1 (en) | 1999-06-10 | 2002-09-17 | 3M Innovative Properties Company | Amide substituted imidazoquinolines |
| US6756382B2 (en) | 1999-06-10 | 2004-06-29 | 3M Innovative Properties Company | Amide substituted imidazoquinolines |
| US6573273B1 (en) | 1999-06-10 | 2003-06-03 | 3M Innovative Properties Company | Urea substituted imidazoquinolines |
| US6541485B1 (en) | 1999-06-10 | 2003-04-01 | 3M Innovative Properties Company | Urea substituted imidazoquinolines |
| US6315985B1 (en) | 1999-06-18 | 2001-11-13 | 3M Innovative Properties Company | C-17/21 OH 20-ketosteroid solution aerosol products with enhanced chemical stability |
| AU783745B2 (en) | 1999-08-13 | 2005-12-01 | Hybridon, Inc. | Modulation of oligonucleotide CpG-mediated immune stimulation by positional modification of nucleosides |
| US6432989B1 (en) | 1999-08-27 | 2002-08-13 | Pfizer Inc | Use of CRF antagonists to treat circadian rhythm disorders |
| CO5271670A1 (en) | 1999-10-29 | 2003-04-30 | Pfizer Prod Inc | ANTIGONISTS OF THE CORTICITROPINE RELEASE FACTOR AND RELATED COMPOSITIONS |
| PT1666028E (en) | 1999-10-29 | 2010-06-15 | Novartis Ag | Dry powder compositions having improved dispersivity |
| US6376669B1 (en) | 1999-11-05 | 2002-04-23 | 3M Innovative Properties Company | Dye labeled imidazoquinoline compounds |
| US6916925B1 (en) | 1999-11-05 | 2005-07-12 | 3M Innovative Properties Co. | Dye labeled imidazoquinoline compounds |
| US6313156B1 (en) | 1999-12-23 | 2001-11-06 | Icos Corporation | Thiazole compounds as cyclic-AMP-specific phosphodiesterase inhibitors |
| US20040023870A1 (en) | 2000-01-21 | 2004-02-05 | Douglas Dedera | Methods of therapy and diagnosis using targeting of cells that express toll-like receptor proteins |
| GB0001704D0 (en) | 2000-01-25 | 2000-03-15 | Glaxo Group Ltd | Protein |
| BR0108303A (en) | 2000-02-09 | 2003-03-05 | Hokuriku Pharmaceutical | 1h-Imidazopyridine Derivatives |
| JP2003528068A (en) | 2000-03-17 | 2003-09-24 | コリクサ コーポレイション | New amphiphilic aldehydes and their use as adjuvants and immune effectors |
| US20010046968A1 (en) | 2000-03-23 | 2001-11-29 | Zagon Ian S. | Opioid growth factor modulates angiogenesis |
| MXPA02009460A (en) | 2000-03-30 | 2003-02-12 | Shionogi & Co | Novel process for producing fused imidazopyridine derivative and novel crystal form. |
| US6894060B2 (en) | 2000-03-30 | 2005-05-17 | 3M Innovative Properties Company | Method for the treatment of dermal lesions caused by envenomation |
| WO2001090323A2 (en) | 2000-05-19 | 2001-11-29 | Millennium Pharmaceuticals, Inc. | 46638, a putative family member of human lipoxygenase |
| DE10020465A1 (en) | 2000-04-26 | 2001-11-08 | Osram Opto Semiconductors Gmbh | Radiation-emitting semiconductor component with luminescence conversion element |
| DE10029580C1 (en) | 2000-06-15 | 2002-01-10 | Ferton Holding Sa | Device for removing body stones with an intracorporeal lithotripter |
| US6387383B1 (en) | 2000-08-03 | 2002-05-14 | Dow Pharmaceutical Sciences | Topical low-viscosity gel composition |
| US6900016B1 (en) | 2000-09-08 | 2005-05-31 | Applera Corporation | Polymorphisms in known genes associated with inflammatory autoimmune disease, methods of detection and uses thereof |
| JP2005500806A (en) | 2000-09-15 | 2005-01-13 | コーリー ファーマシューティカル ゲーエムベーハー | Process for high-throughput screening of immune agonist / immunoantagonists based on CpG |
| US20020055517A1 (en) | 2000-09-15 | 2002-05-09 | 3M Innovative Properties Company | Methods for delaying recurrence of herpes virus symptoms |
| GB0023008D0 (en) | 2000-09-20 | 2000-11-01 | Glaxo Group Ltd | Improvements in vaccination |
| EP1342799B1 (en) | 2000-12-07 | 2007-01-17 | Aoyama Seisakusho Co., Ltd. | Method for baking steel parts |
| US6660735B2 (en) | 2000-12-08 | 2003-12-09 | 3M Innovative Properties Company | Urea substituted imidazoquinoline ethers |
| US6664264B2 (en) | 2000-12-08 | 2003-12-16 | 3M Innovative Properties Company | Thioether substituted imidazoquinolines |
| WO2002046749A2 (en) | 2000-12-08 | 2002-06-13 | 3M Innovative Properties Company | Screening method for identifying compounds that selectively induce interferon alpha |
| US6677347B2 (en) | 2000-12-08 | 2004-01-13 | 3M Innovative Properties Company | Sulfonamido ether substituted imidazoquinolines |
| UA74852C2 (en) | 2000-12-08 | 2006-02-15 | 3M Innovative Properties Co | Urea-substituted imidazoquinoline ethers |
| US6545016B1 (en) | 2000-12-08 | 2003-04-08 | 3M Innovative Properties Company | Amide substituted imidazopyridines |
| US6545017B1 (en) | 2000-12-08 | 2003-04-08 | 3M Innovative Properties Company | Urea substituted imidazopyridines |
| US6677348B2 (en) | 2000-12-08 | 2004-01-13 | 3M Innovative Properties Company | Aryl ether substituted imidazoquinolines |
| US6660747B2 (en) | 2000-12-08 | 2003-12-09 | 3M Innovative Properties Company | Amido ether substituted imidazoquinolines |
| US6664260B2 (en) | 2000-12-08 | 2003-12-16 | 3M Innovative Properties Company | Heterocyclic ether substituted imidazoquinolines |
| US6525064B1 (en) | 2000-12-08 | 2003-02-25 | 3M Innovative Properties Company | Sulfonamido substituted imidazopyridines |
| UA74593C2 (en) | 2000-12-08 | 2006-01-16 | 3M Innovative Properties Co | Substituted imidazopyridines |
| US6667312B2 (en) | 2000-12-08 | 2003-12-23 | 3M Innovative Properties Company | Thioether substituted imidazoquinolines |
| US20020107262A1 (en) | 2000-12-08 | 2002-08-08 | 3M Innovative Properties Company | Substituted imidazopyridines |
| US6664265B2 (en) | 2000-12-08 | 2003-12-16 | 3M Innovative Properties Company | Amido ether substituted imidazoquinolines |
| US20020182274A1 (en) | 2001-03-21 | 2002-12-05 | Kung-Ming Lu | Methods for inhibiting cancer growth, reducing infection and promoting general health with a fermented soy extract |
| ES2314042T3 (en) | 2001-04-17 | 2009-03-16 | Dainippon Sumitomo Pharma Co., Ltd. | NEW DERIVATIVES OF ADENINA. |
| US20020193729A1 (en) | 2001-04-20 | 2002-12-19 | Cormier Michel J.N. | Microprojection array immunization patch and method |
| EP1379552B2 (en) | 2001-04-20 | 2014-11-19 | The Institute for Systems Biology | Toll-like receptor 5 ligands and methods of use |
| US6627639B2 (en) | 2001-04-26 | 2003-09-30 | Wyeth | Uses for indoletetrahydropyridine derivatives of 2,3-dihydro-7H-[1,4]dioxino-[2,3-e]indole |
| US7226928B2 (en) | 2001-06-15 | 2007-06-05 | 3M Innovative Properties Company | Methods for the treatment of periodontal disease |
| US6756399B2 (en) | 2001-06-29 | 2004-06-29 | The United States Of America As Represented By The Department Of Health And Human Services | Use of lipoxygenase inhibitors and PPAR ligands as anti-cancer therapeutic and intervention agents |
| US6987187B2 (en) | 2001-07-16 | 2006-01-17 | Shionogi & Co., Ltd. | Process for preparation of amidine derivatives |
| EP1427445A4 (en) | 2001-08-30 | 2006-09-06 | 3M Innovative Properties Co | Methods of maturing plasmacytoid dendritic cells using immune response modifier molecules |
| US7132438B2 (en) | 2001-10-09 | 2006-11-07 | Amgen Inc. | Benzimidazole derivatives |
| CA2462203A1 (en) | 2001-10-12 | 2003-11-20 | University Of Iowa Research Foundation | Methods and products for enhancing immune responses using imidazoquinoline compounds |
| PT1719511E (en) | 2001-11-16 | 2009-03-06 | Coley Pharm Group Inc | N-[4-(4-amino-2-ethyl-1h-imidazo[4,5-c]quinolin-1-yl)butyl]methanesulfonamide, a pharmaceutical composition comprising the same and use thereof |
| US20050009858A1 (en) | 2001-11-17 | 2005-01-13 | Martinez-Colon Maria I | Imiquimod therapies |
| BR0214407A (en) | 2001-11-27 | 2004-10-19 | Anadys Pharmaceuticals Inc | Compounds, pharmaceutical compositions and method of modulating cytokine immunoactivities |
| NZ532769A (en) | 2001-11-29 | 2005-12-23 | 3M Innovative Properties Co | Pharmaceutical formulations comprising an immune response modifier |
| US6677349B1 (en) | 2001-12-21 | 2004-01-13 | 3M Innovative Properties Company | Sulfonamide and sulfamide substituted imidazoquinolines |
| US6775514B2 (en) | 2002-01-11 | 2004-08-10 | Xerox Corporation | Substrate size monitoring system for use in copier/printers |
| US6525028B1 (en) | 2002-02-04 | 2003-02-25 | Corixa Corporation | Immunoeffector compounds |
| US20050281813A1 (en) | 2002-02-14 | 2005-12-22 | Nuvelo, Inc. | Methods of therapy and diagnosis using targeting of cells that express toll-like receptor proteins |
| US7030129B2 (en) | 2002-02-22 | 2006-04-18 | 3M Innovative Properties Company | Method of reducing and treating UVB-induced immunosuppression |
| US20030185835A1 (en) | 2002-03-19 | 2003-10-02 | Braun Ralph P. | Adjuvant for vaccines |
| US8153141B2 (en) | 2002-04-04 | 2012-04-10 | Coley Pharmaceutical Gmbh | Immunostimulatory G, U-containing oligoribonucleotides |
| EP2108370A1 (en) | 2002-04-30 | 2009-10-14 | Unigen Pharmaceuticals, Inc. | Formulation of a mixture of free-B-ring flavonoids and flavans as a therapeutic agent |
| GB0211649D0 (en) | 2002-05-21 | 2002-07-03 | Novartis Ag | Organic compounds |
| US6743920B2 (en) | 2002-05-29 | 2004-06-01 | 3M Innovative Properties Company | Process for imidazo[4,5-c]pyridin-4-amines |
| AU2003237386A1 (en) | 2002-06-07 | 2003-12-22 | 3M Innovative Properties Company | Ether substituted imidazopyridines |
| US6852861B2 (en) | 2002-07-23 | 2005-02-08 | Biogal Gyogyszergyar Rt. | Preparation of 1H-imidazo [4,5-C] quinolin-4-amines via 1H-imidazo [4,5-C] quinolin-4-phthalimide intermediates |
| KR20050030958A (en) | 2002-07-26 | 2005-03-31 | 비오갈 기오기스제르갸르 알티. | Preparation of 1h-imidazo[4,5-c]quinolin-4-amines via novel 1h-imidazo[4,5-c]quinolin-4-cyano and 1h-imidazo[4,5-c]quinolin-4-carboxamide intermediates |
| CA2495570C (en) | 2002-08-15 | 2012-12-04 | 3M Innovative Properties Company | Immunostimulatory compositions and methods of stimulating an immune response |
| AU2003299082A1 (en) | 2002-09-26 | 2004-04-19 | 3M Innovative Properties Company | 1h-imidazo dimers |
| CA2502429A1 (en) | 2002-10-31 | 2004-05-21 | Amgen Inc. | Antiinflammation agents |
| AU2003294249A1 (en) | 2002-11-08 | 2004-06-03 | Trimeris, Inc. | Hetero-substituted benzimidazole compounds and antiviral uses thereof |
| AU2003287324A1 (en) | 2002-12-11 | 2004-06-30 | 3M Innovative Properties Company | Gene expression systems and recombinant cell lines |
| AU2003287316A1 (en) | 2002-12-11 | 2004-06-30 | 3M Innovative Properties Company | Assays relating to toll-like receptor activity |
| EP2572715A1 (en) | 2002-12-30 | 2013-03-27 | 3M Innovative Properties Company | Immunostimulatory Combinations |
| US7375180B2 (en) | 2003-02-13 | 2008-05-20 | 3M Innovative Properties Company | Methods and compositions related to IRM compounds and Toll-like receptor 8 |
| EP1599726A4 (en) | 2003-02-27 | 2009-07-22 | 3M Innovative Properties Co | Selective modulation of tlr-mediated biological activity |
| EP1601365A4 (en) | 2003-03-04 | 2009-11-11 | 3M Innovative Properties Co | Prophylactic treatment of uv-induced epidermal neoplasia |
| US7163947B2 (en) | 2003-03-07 | 2007-01-16 | 3M Innovative Properties Company | 1-Amino 1H-imidazoquinolines |
| BRPI0408125A (en) | 2003-03-07 | 2006-03-01 | 3M Innovative Properties Co | 1-amino 1h-imidazoquinolines |
| CA2518282C (en) | 2003-03-13 | 2012-11-06 | 3M Innovative Properties Company | Methods of improving skin quality |
| US7179253B2 (en) | 2003-03-13 | 2007-02-20 | 3M Innovative Properties Company | Method of tattoo removal |
| AU2004220466A1 (en) | 2003-03-13 | 2004-09-23 | 3M Innovative Properties Company | Methods for diagnosing skin lesions |
| US20040192585A1 (en) | 2003-03-25 | 2004-09-30 | 3M Innovative Properties Company | Treatment for basal cell carcinoma |
| WO2004087049A2 (en) | 2003-03-25 | 2004-10-14 | 3M Innovative Properties Company | Selective activation of cellular activities mediated through a common toll-like receptor |
| ES2423800T3 (en) | 2003-03-28 | 2013-09-24 | Novartis Vaccines And Diagnostics, Inc. | Use of organic compounds for immunopotentiation |
| WO2004091500A2 (en) | 2003-04-10 | 2004-10-28 | 3M Innovative Properties Company | Delivery of immune response modifier compounds |
| US20040265351A1 (en) | 2003-04-10 | 2004-12-30 | Miller Richard L. | Methods and compositions for enhancing immune response |
| WO2004096144A2 (en) | 2003-04-28 | 2004-11-11 | 3M Innovative Properties Company | Compositions and methods for induction of opioid receptors |
| WO2004110991A2 (en) | 2003-06-06 | 2004-12-23 | 3M Innovative Properties Company | PROCESS FOR IMIDAZO[4,5-c]PYRIDIN-4-AMINES |
| US20050032829A1 (en) | 2003-06-06 | 2005-02-10 | 3M Innovative Properties Company | Process for imidazo[4,5-c]pyridin-4-amines |
| BRPI0411514A (en) | 2003-06-20 | 2006-08-01 | Coley Pharm Gmbh | small molecule toll-like receptor antagonists |
| MY157827A (en) | 2003-06-27 | 2016-07-29 | 3M Innovative Properties Co | Sulfonamide substituted imidazoquinolines |
| US20050106300A1 (en) | 2003-06-30 | 2005-05-19 | Purdue Research Foundation | Method for producing a material having an increased solubility in alcohol |
| JP2007501252A (en) | 2003-08-05 | 2007-01-25 | スリーエム イノベイティブ プロパティズ カンパニー | Formulation containing immune response modifier |
| CA2535117A1 (en) | 2003-08-12 | 2005-03-03 | 3M Innovative Properties Company | Oxime substituted imidazo-containing compounds |
| CA2535338C (en) | 2003-08-14 | 2013-05-28 | 3M Innovative Properties Company | Substituted 1h-imidazo[4,5-c]pyridin-4-amines,1h-imidazo[4,5-c]quinolin -4-amines and 1h-imidazo[4,5-c]naphthyridin-4-amines as immune response modifiers |
| WO2005018574A2 (en) | 2003-08-25 | 2005-03-03 | 3M Innovative Properties Company | Immunostimulatory combinations and treatments |
| EP1658076B1 (en) | 2003-08-27 | 2013-03-06 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted imidazoquinolines |
| CA2537763A1 (en) | 2003-09-05 | 2005-03-17 | 3M Innovative Properties Company | Treatment for cd5+ b cell lymphoma |
| AP2006003542A0 (en) | 2003-09-05 | 2006-04-30 | Anadys Pharmaceuticals Inc | Administration of TLR7 ligands and prodrugs for treatment of infection by hepatitis C virus. |
| GB0321615D0 (en) | 2003-09-15 | 2003-10-15 | Glaxo Group Ltd | Improvements in vaccination |
| US20050059072A1 (en) | 2003-09-17 | 2005-03-17 | 3M Innovative Properties Company | Selective modulation of TLR gene expression |
| WO2005033049A2 (en) | 2003-10-01 | 2005-04-14 | Taro Pharmaceuticals U.S.A., Inc. | METHOD OF PREPARING 4-AMINO-1H-IMIDAZO(4,5-c)QUINOLINES AND ACID ADDITION SALTS THEREOF |
| EP1673087B1 (en) | 2003-10-03 | 2015-05-13 | 3M Innovative Properties Company | Alkoxy substituted imidazoquinolines |
| US20090075980A1 (en) | 2003-10-03 | 2009-03-19 | Coley Pharmaceutical Group, Inc. | Pyrazolopyridines and Analogs Thereof |
| US7544697B2 (en) | 2003-10-03 | 2009-06-09 | Coley Pharmaceutical Group, Inc. | Pyrazolopyridines and analogs thereof |
| CA2543685A1 (en) | 2003-10-31 | 2005-05-12 | 3M Innovative Properties Company | Neutrophil activation by immune response modifier compounds |
| US20050239733A1 (en) | 2003-10-31 | 2005-10-27 | Coley Pharmaceutical Gmbh | Sequence requirements for inhibitory oligonucleotides |
| ITMI20032121A1 (en) | 2003-11-04 | 2005-05-05 | Dinamite Dipharma Spa In Forma Abbr Eviata Dipharm | PROCEDURE FOR THE PREPARATION OF IMIQUIMOD AND ITS INTERMEDIATES |
| US8598192B2 (en) | 2003-11-14 | 2013-12-03 | 3M Innovative Properties Company | Hydroxylamine substituted imidazoquinolines |
| US7897767B2 (en) | 2003-11-14 | 2011-03-01 | 3M Innovative Properties Company | Oxime substituted imidazoquinolines |
| CU23404A1 (en) | 2003-11-19 | 2009-08-04 | Ct Ingenieria Genetica Biotech | NEISSERIA MENINGITIDIS CAPSULAR POLYSACARIDS AS MUCOSOPT IMMUNOPOTENTIZERS AND RESULTING FORMULATIONS |
| AR046845A1 (en) | 2003-11-21 | 2005-12-28 | Novartis Ag | DERIVATIVES OF 1H-IMIDAZO [4,5-C] QUINOLINE FOR THE TREATMENT OF PROTEIN-KINASE DEPENDENT DISEASES |
| WO2005054237A1 (en) | 2003-11-21 | 2005-06-16 | Novartis Ag | 1h-imidazoquinoline derivatives as protein kinase inhibitors |
| EP1686992A4 (en) | 2003-11-25 | 2009-11-04 | 3M Innovative Properties Co | Hydroxylamine and oxime substituted imidazoquinolines, imidazopyridines, and imidazonaphthyridines |
| RU2409576C2 (en) | 2003-11-25 | 2011-01-20 | 3М Инновейтив Пропертиз Компани | Systems containing imidazole ring with substitutes, and methods of obtaining said systems |
| WO2005055932A2 (en) | 2003-12-02 | 2005-06-23 | 3M Innovative Properties Company | Therapeutic combinations and methods including irm compounds |
| US20050226878A1 (en) | 2003-12-02 | 2005-10-13 | 3M Innovative Properties Company | Therapeutic combinations and methods including IRM compounds |
| JP2007513170A (en) | 2003-12-04 | 2007-05-24 | スリーエム イノベイティブ プロパティズ カンパニー | Sulfone substituted imidazo ring ether |
| US7888349B2 (en) | 2003-12-29 | 2011-02-15 | 3M Innovative Properties Company | Piperazine, [1,4]Diazepane, [1,4]Diazocane, and [1,5]Diazocane fused imidazo ring compounds |
| EP1701955A1 (en) | 2003-12-29 | 2006-09-20 | 3M Innovative Properties Company | Arylalkenyl and arylalkynyl substituted imidazoquinolines |
| WO2005065678A1 (en) | 2003-12-30 | 2005-07-21 | 3M Innovative Properties Company | Immunomodulatory combinations |
| US8735421B2 (en) | 2003-12-30 | 2014-05-27 | 3M Innovative Properties Company | Imidazoquinolinyl sulfonamides |
| WO2005067500A2 (en) | 2003-12-30 | 2005-07-28 | 3M Innovative Properties Company | Enhancement of immune responses |
| CA2559863A1 (en) | 2004-03-24 | 2005-10-13 | 3M Innovative Properties Company | Amide substituted imidazopyridines, imidazoquinolines, and imidazonaphthyridines |
| US20070166384A1 (en) | 2004-04-09 | 2007-07-19 | Zarraga Isidro Angelo E | Methods , composition and preparations for delivery of immune response modifiers |
| US20050267145A1 (en) | 2004-05-28 | 2005-12-01 | Merrill Bryon A | Treatment for lung cancer |
| US20080015184A1 (en) | 2004-06-14 | 2008-01-17 | 3M Innovative Properties Company | Urea Substituted Imidazopyridines, Imidazoquinolines, and Imidazonaphthyridines |
| US8017779B2 (en) | 2004-06-15 | 2011-09-13 | 3M Innovative Properties Company | Nitrogen containing heterocyclyl substituted imidazoquinolines and imidazonaphthyridines |
| US7897609B2 (en) | 2004-06-18 | 2011-03-01 | 3M Innovative Properties Company | Aryl substituted imidazonaphthyridines |
| WO2006009832A1 (en) | 2004-06-18 | 2006-01-26 | 3M Innovative Properties Company | Substituted imidazo ring systems and methods |
| WO2006065280A2 (en) | 2004-06-18 | 2006-06-22 | 3M Innovative Properties Company | Isoxazole, dihydroisoxazole, and oxadiazole substituted imidazo ring compounds and methods |
| US20070259907A1 (en) | 2004-06-18 | 2007-11-08 | Prince Ryan B | Aryl and arylalkylenyl substituted thiazoloquinolines and thiazolonaphthyridines |
| US8026366B2 (en) | 2004-06-18 | 2011-09-27 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted thiazoloquinolines and thiazolonaphthyridines |
| PL1789042T3 (en) | 2004-09-02 | 2012-09-28 | 3M Innovative Properties Co | 1-alkoxy 1h-imidazo ring systems and methods |
| WO2006026760A2 (en) | 2004-09-02 | 2006-03-09 | 3M Innovative Properties Company | 1-amino imidazo-containing compounds and methods |
| CA2578975A1 (en) | 2004-09-02 | 2006-03-16 | 3M Innovative Properties Company | 2-amino 1h imidazo ring systems and methods |
| WO2006028451A1 (en) | 2004-09-03 | 2006-03-16 | 3M Innovative Properties Company | 1-amino 1-h-imidazoquinolines |
| JP4769810B2 (en) | 2004-09-14 | 2011-09-07 | ノバルティス ヴァクシンズ アンド ダイアグノスティクス, インコーポレイテッド | Imidazoquinoline compounds |
| EP1819226A4 (en) | 2004-12-08 | 2010-12-29 | 3M Innovative Properties Co | Immunostimulatory combinations and methods |
| US20110070575A1 (en) | 2004-12-08 | 2011-03-24 | Coley Pharmaceutical Group, Inc. | Immunomodulatory Compositions, Combinations and Methods |
| AU2005322843B2 (en) | 2004-12-30 | 2012-03-08 | 3M Innovative Properties Company | Immune response modifier formulations and methods |
| JP5313502B2 (en) | 2004-12-30 | 2013-10-09 | スリーエム イノベイティブ プロパティズ カンパニー | Substituted chiral condensed [1,2] imidazo [4,5-c] cyclic compounds |
| AU2005322898B2 (en) | 2004-12-30 | 2011-11-24 | 3M Innovative Properties Company | Chiral fused (1,2)imidazo(4,5-c) ring compounds |
| US9248127B2 (en) | 2005-02-04 | 2016-02-02 | 3M Innovative Properties Company | Aqueous gel formulations containing immune response modifiers |
| AU2006212765B2 (en) | 2005-02-09 | 2012-02-02 | 3M Innovative Properties Company | Alkyloxy substituted thiazoloquinolines and thiazolonaphthyridines |
| WO2007120121A2 (en) | 2005-02-09 | 2007-10-25 | Coley Pharmaceutical Group, Inc. | Oxime and hydroxylamine substituted thiazolo[4,5-c] ring compounds and methods |
| US7968563B2 (en) | 2005-02-11 | 2011-06-28 | 3M Innovative Properties Company | Oxime and hydroxylamine substituted imidazo[4,5-c] ring compounds and methods |
| WO2006086633A2 (en) | 2005-02-11 | 2006-08-17 | Coley Pharmaceutical Group, Inc. | Substituted fused [1,2]imidazo[4,5-c] ring compounds and methods |
| JP2008532933A (en) | 2005-02-11 | 2008-08-21 | コーリー ファーマシューティカル グループ,インコーポレイテッド | Substituted imidazoquinolines and substituted imidazonaphthyridines |
| JP2008543725A (en) | 2005-02-23 | 2008-12-04 | コーリー ファーマシューティカル グループ,インコーポレイテッド | Hydroxyalkyl substituted imidazoquinolines |
| WO2006091567A2 (en) | 2005-02-23 | 2006-08-31 | Coley Pharmaceutical Group, Inc. | Hydroxyalkyl substituted imidazoquinoline compounds and methods |
| AU2006216686A1 (en) | 2005-02-23 | 2006-08-31 | Coley Pharmaceutical Group, Inc. | Method of preferentially inducing the biosynthesis of interferon |
| CA2598639A1 (en) | 2005-02-23 | 2006-08-31 | Coley Pharmaceutical Group, Inc. | Hydroxyalkyl substituted imidazonaphthyridines |
| WO2006121528A2 (en) | 2005-04-01 | 2006-11-16 | Coley Pharmaceutical Group, Inc. | Ring closing and related methods and intermediates |
| AU2006232294A1 (en) | 2005-04-01 | 2006-10-12 | Coley Pharmaceutical Group, Inc. | Pyrazolo[3,4-c]quinolines, pyrazolo[3,4-c]naphthyridines, analogs thereof, and methods |
| AU2006232377A1 (en) | 2005-04-01 | 2006-10-12 | Coley Pharmaceutical Group, Inc. | Pyrazolopyridine-1,4-diamines and analogs thereof |
| US7943636B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | 1-substituted pyrazolo (3,4-C) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
| GB0510390D0 (en) | 2005-05-20 | 2005-06-29 | Novartis Ag | Organic compounds |
| BRPI0615250A2 (en) | 2005-09-02 | 2011-05-10 | Pfizer | Hydroxy substituted 1h-imidazopyridines, pharmaceutical composition containing same, as well as their uses |
| US8476292B2 (en) | 2005-09-09 | 2013-07-02 | 3M Innovative Properties Company | Amide and carbamate derivatives of N-{2-[4-amino-2-(ethoxymethyl)-1H-imidazo[4,5-c] quinolin-1-Yl]-1,1-dimethylethyl}methanesulfonamide and methods |
| ZA200803029B (en) | 2005-09-09 | 2009-02-25 | Coley Pharm Group Inc | Amide and carbamate derivatives of alkyl substituted /V-[4-(4-amino-1H-imidazo[4,5-c] quinolin-1-yl)butyl] methane-sulfonamides and methods |
| CA2623541A1 (en) | 2005-09-23 | 2007-03-29 | Coley Pharmaceutical Group, Inc. | Method for 1h-imidazo[4,5-c]pyridines and analogs thereof |
| EP1948173B1 (en) | 2005-11-04 | 2013-07-17 | 3M Innovative Properties Company | Hydroxy and alkoxy substituted 1h-imidazoquinolines and methods |
| WO2007062043A1 (en) | 2005-11-23 | 2007-05-31 | Coley Pharmaceutical Group Inc. | Method of activating murine toll-like receptor 8 |
| KR20080077982A (en) | 2005-12-16 | 2008-08-26 | 콜레이 파마시티컬 그룹, 인코포레이티드 | Substituted imidazoquinolines, imidazonaphthyridines, and imidazopyridines, compositions, and methods |
| WO2007079202A2 (en) | 2005-12-28 | 2007-07-12 | Coley Pharmaceutical Group, Inc. | Treatment for acute lymhoblastic leukemia |
| WO2007079203A2 (en) | 2005-12-28 | 2007-07-12 | 3M Innovative Properties Company | Treatment for cutaneous t cell lymphoma |
| WO2007079171A2 (en) | 2005-12-28 | 2007-07-12 | Coley Pharmaceutical Group, Inc. | Treatment for hodgkin's lymphoma |
| WO2007079086A1 (en) | 2005-12-28 | 2007-07-12 | Coley Pharmaceutical Group, Inc. | Pyrazoloalkyl substituted imidazo ring compounds and methods |
| WO2007079169A2 (en) | 2005-12-28 | 2007-07-12 | Coley Pharmaceutical Group, Inc. | Treatment for acute myeloid leukemia |
| WO2007079146A1 (en) | 2005-12-28 | 2007-07-12 | Coley Pharmaceutical Group, Inc | Treatment for non-hodgkin's lymphoma |
| WO2007092641A2 (en) | 2006-02-10 | 2007-08-16 | Coley Pharmaceutical Group, Inc. | Method for substituted 1h-imidazo[4,5-c]pyridines |
| US8088788B2 (en) | 2006-03-15 | 2012-01-03 | 3M Innovative Properties Company | Substituted fused[1,2] imidazo[4,5-c] ring compounds and methods |
| WO2007106854A2 (en) | 2006-03-15 | 2007-09-20 | Coley Pharmaceutical Group, Inc. | Hydroxy and alkoxy substituted 1h-imidazonaphthyridines and methods |
| WO2007143526A2 (en) | 2006-06-05 | 2007-12-13 | Coley Pharmaceutical Group, Inc. | Substituted tetrahydroimidazonaphthyridines and methods |
| WO2008002646A2 (en) | 2006-06-27 | 2008-01-03 | Schering-Plough Healthcare Products, Inc. | Aerosol lotion formulations |
| US7906506B2 (en) | 2006-07-12 | 2011-03-15 | 3M Innovative Properties Company | Substituted chiral fused [1,2] imidazo [4,5-c] ring compounds and methods |
| US8178539B2 (en) | 2006-09-06 | 2012-05-15 | 3M Innovative Properties Company | Substituted 3,4,6,7-tetrahydro-5H-1,2a,4a,8-tetraazacyclopenta[cd]phenalenes and methods |
| WO2008036312A1 (en) | 2006-09-19 | 2008-03-27 | Coley Pharmaceutical Group, Inc. | Fungicidal methods using immune response modifier compounds |
| WO2008045543A1 (en) | 2006-10-13 | 2008-04-17 | Coley Pharmaceutical Group, Inc. | Substituted 4h-imidazo [4, 5, 1-ij] [1, 6] naphthyridine-9-amines and their pharmaceutical use |
-
2006
- 2006-03-31 US US11/887,492 patent/US7943636B2/en not_active Expired - Fee Related
- 2006-03-31 JP JP2008504494A patent/JP2008538550A/en not_active Withdrawn
- 2006-03-31 EP EP06749140A patent/EP1863814A1/en not_active Withdrawn
- 2006-03-31 AU AU2006232375A patent/AU2006232375A1/en not_active Abandoned
- 2006-03-31 CA CA002602590A patent/CA2602590A1/en not_active Abandoned
- 2006-03-31 WO PCT/US2006/012263 patent/WO2006107851A1/en active Application Filing
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4689338A (en) * | 1983-11-18 | 1987-08-25 | Riker Laboratories, Inc. | 1H-Imidazo[4,5-c]quinolin-4-amines and antiviral use |
| WO1995002598A1 (en) * | 1993-07-15 | 1995-01-26 | Minnesota Mining And Manufacturing Company | Fused cycloalkylimidazopyridines |
| EP1104764A1 (en) * | 1998-08-12 | 2001-06-06 | Hokuriku Seiyaku Co., Ltd. | 1h-imidazopyridine derivatives |
| WO2004058759A1 (en) * | 2002-12-20 | 2004-07-15 | 3M Innovative Properties Company | Aryl / hetaryl substituted imidazoquinolines |
| WO2005079195A2 (en) * | 2003-10-03 | 2005-09-01 | 3M Innovative Properties Company | Pyrazolopyridines and analogs thereof |
| WO2006028545A2 (en) * | 2004-06-18 | 2006-03-16 | 3M Innovative Properties Company | Substituted imidazoquinolines, imidazopyridines, and imidazonaphthyridines |
Cited By (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8673932B2 (en) | 2003-08-12 | 2014-03-18 | 3M Innovative Properties Company | Oxime substituted imidazo-containing compounds |
| US7897597B2 (en) | 2003-08-27 | 2011-03-01 | 3M Innovative Properties Company | Aryloxy and arylalkyleneoxy substituted imidazoquinolines |
| US7923429B2 (en) | 2003-09-05 | 2011-04-12 | 3M Innovative Properties Company | Treatment for CD5+ B cell lymphoma |
| US8871782B2 (en) | 2003-10-03 | 2014-10-28 | 3M Innovative Properties Company | Alkoxy substituted imidazoquinolines |
| US7879849B2 (en) | 2003-10-03 | 2011-02-01 | 3M Innovative Properties Company | Pyrazolopyridines and analogs thereof |
| US8598192B2 (en) | 2003-11-14 | 2013-12-03 | 3M Innovative Properties Company | Hydroxylamine substituted imidazoquinolines |
| US7897767B2 (en) | 2003-11-14 | 2011-03-01 | 3M Innovative Properties Company | Oxime substituted imidazoquinolines |
| US8691837B2 (en) | 2003-11-25 | 2014-04-08 | 3M Innovative Properties Company | Substituted imidazo ring systems and methods |
| US8802853B2 (en) | 2003-12-29 | 2014-08-12 | 3M Innovative Properties Company | Arylalkenyl and arylalkynyl substituted imidazoquinolines |
| US8735421B2 (en) | 2003-12-30 | 2014-05-27 | 3M Innovative Properties Company | Imidazoquinolinyl sulfonamides |
| US7915281B2 (en) | 2004-06-18 | 2011-03-29 | 3M Innovative Properties Company | Isoxazole, dihydroisoxazole, and oxadiazole substituted imidazo ring compounds and method |
| US8034938B2 (en) | 2004-12-30 | 2011-10-11 | 3M Innovative Properties Company | Substituted chiral fused [1,2]imidazo[4,5-c] ring compounds |
| US7943609B2 (en) | 2004-12-30 | 2011-05-17 | 3M Innovative Proprerties Company | Chiral fused [1,2]imidazo[4,5-C] ring compounds |
| US9248127B2 (en) | 2005-02-04 | 2016-02-02 | 3M Innovative Properties Company | Aqueous gel formulations containing immune response modifiers |
| US10071156B2 (en) | 2005-02-04 | 2018-09-11 | 3M Innovative Properties Company | Aqueous gel formulations containing immune response modifiers |
| US7968563B2 (en) | 2005-02-11 | 2011-06-28 | 3M Innovative Properties Company | Oxime and hydroxylamine substituted imidazo[4,5-c] ring compounds and methods |
| US7943636B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | 1-substituted pyrazolo (3,4-C) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases |
| US7943610B2 (en) | 2005-04-01 | 2011-05-17 | 3M Innovative Properties Company | Pyrazolopyridine-1,4-diamines and analogs thereof |
| US8951528B2 (en) | 2006-02-22 | 2015-02-10 | 3M Innovative Properties Company | Immune response modifier conjugates |
| EP3085373A1 (en) | 2006-02-22 | 2016-10-26 | 3M Innovative Properties Company | Immune response modifier conjugates |
| US7906506B2 (en) | 2006-07-12 | 2011-03-15 | 3M Innovative Properties Company | Substituted chiral fused [1,2] imidazo [4,5-c] ring compounds and methods |
| US10005772B2 (en) | 2006-12-22 | 2018-06-26 | 3M Innovative Properties Company | Immune response modifier compositions and methods |
| US10144735B2 (en) | 2006-12-22 | 2018-12-04 | 3M Innovative Properties Company | Immune response modifier compositions and methods |
| TWI557121B (en) * | 2011-04-21 | 2016-11-11 | 原真股份有限公司 | Novel kinase inhibitors |
| US9499535B2 (en) | 2011-04-21 | 2016-11-22 | Origenis Gmbh | Kinase inhibitors |
| EP3153180A1 (en) | 2011-06-03 | 2017-04-12 | 3M Innovative Properties Company | Heterobifunctional linkers with polyethylene glycol segments and immune response modifier conjugates made therefrom |
| US10000482B2 (en) | 2012-10-19 | 2018-06-19 | Origenis Gmbh | Kinase inhibitors |
| US10752624B2 (en) | 2012-10-19 | 2020-08-25 | Origenis Gmbh | Kinase inhibitors |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2602590A1 (en) | 2006-10-12 |
| EP1863814A1 (en) | 2007-12-12 |
| US20090163533A1 (en) | 2009-06-25 |
| US7943636B2 (en) | 2011-05-17 |
| JP2008538550A (en) | 2008-10-30 |
| AU2006232375A1 (en) | 2006-10-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7943636B2 (en) | 1-substituted pyrazolo (3,4-C) ring compounds as modulators of cytokine biosynthesis for the treatment of viral infections and neoplastic diseases | |
| JP5247458B2 (en) | Hydroxy and alkoxy substituted 1H-imidazoquinolines and methods | |
| US7943610B2 (en) | Pyrazolopyridine-1,4-diamines and analogs thereof | |
| JP5128940B2 (en) | Substituted imidazoquinolines, imidazopyridines, and imidazonaphthyridines | |
| US8329721B2 (en) | Hydroxy and alkoxy substituted 1H-imidazonaphthyridines and methods | |
| WO2006026760A2 (en) | 1-amino imidazo-containing compounds and methods | |
| WO2007079086A1 (en) | Pyrazoloalkyl substituted imidazo ring compounds and methods | |
| WO2007143526A2 (en) | Substituted tetrahydroimidazonaphthyridines and methods | |
| WO2006074003A2 (en) | CHIRAL FUSED [1,2]IMIDAZO[4,5-c] RING COMPOUNDS | |
| EP1784180A2 (en) | 2-amino 1h imidazo ring systems and methods | |
| JP2009519955A (en) | Substituted imidazoquinolines, imidazonaphthyridines and imidazopyridines, compositions and methods | |
| WO2006065280A2 (en) | Isoxazole, dihydroisoxazole, and oxadiazole substituted imidazo ring compounds and methods | |
| EP1924581A1 (en) | Hydroxy substituted 1h-imidazopyridines and methods | |
| WO2006038923A2 (en) | Aryl substituted imidazonaphthyridines | |
| WO2006009826A1 (en) | Aryloxy and arylalkyleneoxy substituted thiazoloquinolines and thiazolonaphthyridines | |
| WO2006086634A2 (en) | Oxime and hydroxylamine substituted imidazo[4,5-c] ring compounds and methods | |
| WO2006086633A2 (en) | Substituted fused [1,2]imidazo[4,5-c] ring compounds and methods | |
| US9550773B2 (en) | Substituted imidazoquinolines, imidazopyridines, and imidazonaphthyridines |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| ENP | Entry into the national phase |
Ref document number: 2602590 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006232375 Country of ref document: AU |
|
| ENP | Entry into the national phase |
Ref document number: 2008504494 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006749140 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2006232375 Country of ref document: AU Date of ref document: 20060331 Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: RU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 11887492 Country of ref document: US |