Abstract
Arsenotučekite, Ni18Sb3AsS16, is a new mineral discovered in the abandoned chromium mine of Tsangli, located in the eastern portion of the Othrys ophiolite complex, central Greece. Tsangli is one of the largest chromite deposit at which chromite was mined since 1870. The Tsangli chromitite occurs as lenticular and irregular bodies. The studied chromitites are hosted in a strongly serpentinized mantle peridotite. Arsenotučekite forms anhedral to subhedral grains that vary in size between 5 μm up to 100 μm, and occurs as single phase grains or is associated with pentlandite, breithauptite, gersdorffite and chlorite. It is brittle and has a metallic luster. In plane-polarized light, it is creamy-yellow, the bireflectance is barely perceptible and the pleochroism is weak. In crossed polarized reflected light, the anisotropic rotation tints vary from pale blue to brown. Internal reflections were not observed. Reflectance values of arsenotučekite in air (Ro, Re′ in %) are: 41.8–46.4 at 470 nm, 47.2–50.6 at 546 nm, 49.4–52.3 at 589 nm, and 51.3–53.2 at 650 nm. The empirical formula of arsenotučekite, based on 38 atoms per formula unit, and according to the structural results, is (Ni16.19Co1.01Fe0.83)Σ18.03Sb3(As0.67Sb0.32)Σ0.99S15.98. The mass density is 6.477 g·cm−3. The simplified chemical formula is (Ni,Co,Fe)18Sb3(As,Sb)S16. The mineral is tetragonal and belongs to space group I4/mmm, with a = 9.7856(3) Å, c = 10.7582(6) Å, V = 1030.2(6) Å3 and Z = 2. The structure is layered (stacking along the c-axis) and is dominated by three different Ni-coordination polyhedral, one octahedral and two cubic. The arsenotučekite structure can be considered as a superstructure of tučekite resulting from the ordering of Sb and As. The name of the new mineral species indicates the As-dominant of tučekite. Arsenotučekite occurs as rims partly replacing pentlandite and irregularly developed grains. Furthermore, it is locally associated with chlorite. These observations suggest that it was likely precipitated at relatively low temperatures during: 1) the late hydrothermal stages of the ore-forming process by reaction of Sb- and As-bearing solutions with magmatic sulfides such as pentlandite, or 2) during the serpentinization of the host peridotite. The mineral and its name have been approved by the Commission of New Minerals, Nomenclature, and Classification of the International Mineralogical Association (number 2019–135).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, three new minerals, namely tsikourasite, Mo3Ni2P1 + x (x < 0.25), grammatikopoulosite, NiVP, and eliopoulosite, V7S8, were discovered in the heavy mineral concentrates from chromitite samples collected in the Othrys ophiolite, central Greece (Zaccarini et al. 2019; Bindi et al. 2020a, b). The Othrys ophiolite is structurally divided into west and east Othrys occurrences, which probably formed in different geotectonic environments. The western type includes formations, which are related to an extension regime, i.e. back-arc basin or mid-ocean ridge (MOR) (Barth et al. 2003, 2008; Barth and Gluhak 2009; Dijkstra et al. 2003), while the Othrys ophiolite from the eastern region is associated with a supra-subduction zone setting (SSZ), as proposed by Barth and Gluhak (2009) and Magganas and Koutsovitis (2015).
The three new minerals were found in the abandoned chromium mine of Agios Stefanos, located in the west area of the Othrys complex. Due to the promising mineralogical results, an additional amount of chromite was collected from the Tsangli mining area located in the east domain of the Othrys complex. It was studied by a combination of chemical analysis and X-ray diffraction techniques.
An additional new mineral was then discovered and approved by the Commission of New Minerals, Nomenclature and Classification of the International Mineralogical Association (number 2019–135). The simplified chemical formula of the new mineral is (Ni,Co,Fe)18Sb3(As,Sb)S16 and it has been named arsenotučekite to indicate the As-dominant tučekite, as previously proposed for arsenohauchecornite, the As-hauchecornite (Gait and Harris 1980; Grice and Ferguson 1989). Holotype material is deposited in the collections of the Natural History Museum, London (catalogue number BM 2020,1).
Geological background, description of the chromitite and chromite composition
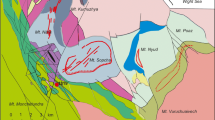
Arsenotučekite has been found in massive chromitite which was collected from the Tsangli mining area located in the east Othrys ophiolite, central Greece (Fig. 1a, b). The Othrys ophiolite is a remnant of the Tethys Ocean, which extended between Eurasia and Gondwanaland in the Jurassic.
The geological history of the Othrys ophiolite is still debated and several models have been proposed for its origin. In particular, the east Othrys ophiolite is considered to have formed in the island arc of a SSZ geo-dynamic setting (Barth and Gluhak 2009; Magganas and Koutsovitis 2015). In contrast, the west Othrys ophiolite represents a section of MOR-type oceanic lithosphere formed along a paleo-transform fault (Rassios and Konstantopoulou 1993; Dijkstra et al. 2003).
The Othrys ophiolite is strongly tectonically overprinted and fragmented, but an almost complete stratigraphy was recognized. It comprises a mantle peridotite, ultramafic and gabbroic cumulates, sheeted dikes, pillow lavas and a sedimentary cover (Rassios and Konstantopoulou 1993; Bortolotti et al. 2008; Koutsovitis 2012).
Although the mantle rocks of the Othrys ophiolite show characteristics of MOR and SSZ geodynamic settings, the occurrence of chromitites was reported in both of the mantle peridotites. The Othrys peridotites consist of fertile plagioclase lherzolites in the western part of the complex and of depleted harzburgites in the eastern domain. They represent residual rocks characterized by a different degree of partial melting.
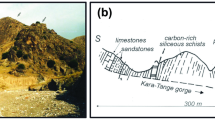
The studied sample was collected from the Tsangli mining area located in the eastern portion of the Otrhys complex, about 40 km North-East of the Domokos village (Fig. 1b) and 1 km North-East of the Eretria village (Fig. 1c).
The sample that hosts arsenotučekite is a massive chromite host in an altered mantle peridotite. The chromitite contains interstitial Ni-Cu-Fe sulfides, such as pentlandite, and subordinate pyrrhotite and chalcopyrite (Economou and Naldrett 1984). The serpentinized peridotites are over- and underlain by schist, covered in turn unconformably by Cenomanian limestone (Economou and Naldrett 1984) (Fig. 1c). Conglomerates are widespread in the mining area (Fig. 1c).
Following the procedure described by Ifandi et al. (2018), chemical analyses of the spinels obtained on chromitite yielded the following compositional range: Cr2O3 (43.39–51.14 wt%), Al2O3 (15.26–22.32 wt%), MgO (11.77–14.08 wt%), and FeO (13.56–16.31 wt%). Fe2O3, calculated on the basis of the spinel stoichiometry, ranges from 3.46 to 6.92 wt%. Minor elements are MnO (0.00–0.09 wt%), ZnO (0.00–0.08 wt%), V2O3 (0.08–0.19 wt%) and NiO (0.10–0.23 wt%). The TiO2 content is low (0.04–0.16 wt%), and consistent with most mantle-hosted podiform chromitites.
Previous investigations had shown that the Tsangli chromitites contain several platinum group minerals (PGM), including laurite, erlichmanite, alloys in the Os-Ir-Ru system, irarsite, ruasrsite, osarsite, sperrylite and merenskyite (Tsikouras et al. 2016). Most of the discovered PGM have a magmatic origin and only few of them were re-worked and altered during the serpentinization (Tsikouras et al. 2016).
Samples and experimental
Concentrate specimens of heavy minerals were prepared from samples of massive chromitite weighing ca. 10 kg. The processing and recovery were carried out following the procedure described elsewhere (Tsikouras et al. 2016; Ifandi et al. 2018; Zaccarini et al. 2019; Bindi et al. 2020a, b). The heavy minerals were embedded in epoxy, and ground and polished for mineralogical investigation.
Quantitative chemical analyses of arsenotučekite were obtained using a JEOL JXA-8200 electron probe micro-analyser, operating in WDS (wavelength dispersive X-ray spectrometry) mode. Major and minor elements were determined at 20 kV accelerating voltage and 10 nA beam current, with 20 s as counting time for the peaks and 10 s for the backgrounds. The beam diameter was about 1 μm in size. For the WDS analyses, the following lines and diffracting crystals were used: S = Kα, PETJ, As = Lα, TAP, Sb = Lα, PETJ, and Fe, Co, Ni, = Kα, LIFH. The following reference materials were selected: skutterudite (Co, As), stibnite (Sb, S), millerite (Ni), and pyrite (Fe). The same instrument was used to obtain back-scattered electron (BSE) images.
Reflectance measurements were made using a J & M TIDAS diode array spectrometer attached to a Zeiss Axiotron microscope. Measurements were made in air relative to a WTiC standard.
Single-crystal X-ray analysis was done on a crystal fragment hand-picked from the polished section under a reflected light microscope. The crystal (about 35 μm in size) was carefully washed in acetone several times. It did not show any visible other phase attached to the surface. The analysis was carried out using a Bruker D8 Venture Photon 100 CMOS diffractometer system, using graphite-monochromatized MoKα radiation (λ = 0.71073 Å). X-ray powder diffraction data were not collected owing to the small size of the grain and the limited material available.
Physical and optical properties
More than 50 grains of arsenotučekite were identified in the studied polished sections. Arsenotučekite forms anhedral to subhedral grains and ranges in size from 5 μm to rarely up to about 100 μm (Fig. 2a–d). It occurs as single phase grains (Fig. 2a) but was also associated with chlorite (Fig. 2b), pentlandite, breithauptite (Fig. 2c, d) and gersdorffite. It is brittle and has a metallic luster.
In plane-polarized light, the color is creamy-yellow (Fig. 2a–c), the bireflectance is barely perceptible and the pleochroism is weak. In crossed polarized reflected light, the anisotropic rotation tints vary from pale blue to brown. Internal reflections were not observed.
Reflectance values in air (R in %) requested by the Commission of Ore Mineralogy (COM) of arsenotučekite are (Ro, Re′ in %) are: 41.8–46.4 at 470 nm, 47.2–50.6 at 546 nm, 49.4–52.3 at 589 nm, and 51.3–53.2 at 650 nm. The reflectance values are reported in Table 1 and Fig. 3.
Mass density was not measured because of the small amount of available material. The mass density, on the basis of the ideal chemical formula and unit-cell volume from single-crystal X-ray data, is 6.477 g·cm−3. The calculated mass density is equal to 7.085 g·cm−3, based on the empirical composition and unit-cell volume refined from single-crystal XRD data.
Chemical composition and X-ray crystallography
Representative results of chemical analyses of arsenotučekite are listed in Table 2. The empirical formula of arsenotučekite, based on 38 atoms and according to the structural results (see below), is (Ni16.19Co1.01Fe0.83)Σ18.03Sb3(As0.67Sb0.32)Σ0.99S15.98 and the simplified formula is (Ni,Co,Fe)18Sb3(As,Sb)S16. The ideal formula is Ni18Sb3AsS16, which requires Ni 52.58 wt%, Sb 18.17 wt%, As 3.73 wt% and S 25.52 wt%, in order to sum up to 100 wt%.
Single-crystal X-ray diffraction intensity data were integrated and corrected for Lorentz and polarization factors and absorption using the software package APEX3 (Bruker 2016). A total of 678 unique reflections were collected. Given the similarity in unit-cell values and space groups, the structure was refined starting from the atomic coordinates reported for the I4/mmm crystal structure of arsenohauchecornite (Grice and Ferguson 1989) using the program Shelxl-97 (Sheldrick 2008).
The site occupancy factor at the Ni, Sb and As sites was allowed to vary (Ni versus structural vacancy, Sb versus As) using scattering curves for neutral atoms taken from Wilson (1992). Ni and Sb sites were found to be fully occupied by Ni and Sb, respectively. The mean electron number refined at the As site was 39.1 e−, in excellent agreement with the site population obtained from the chemical data (i.e., As0.65Sb0.35). At the last stage, with anisotropic displacement parameters for all the atoms, the structure was refined to R1 = 0.0562 using 678 independent reflections. Final atomic coordinates and equivalent isotropic displacement parameters are given in Table 3. These data were used to calculate a theoretical X-ray powder diffraction pattern (Table 4). Selected bond distances are shown in Table 5.
The structure of arsenotučekite (Fig. 4) consists of alternating layers of Ni atoms and S, Sb and As atoms, parallel to (001), at approximately one-eighth intervals of the c cell dimension. The structure is dominated by three different Ni coordination polyhedra, two of them are distorted cubes and the third is an octahedron. The Ni1-octahedron has a plane of four S atoms parallel to (001) and two apical Sb atoms. Ni2 and Ni3 are 8-coordinated distorted cubes. Although the latter two sites are similar, Ni2 exhibits a shorter mean bond distance than Ni3. Both of these polyhedra contain a nearly square plane of four atoms, which is a diagonal within the distorted cube.
As shown in Fig. 5, arsenotučekite structure can be considered as a superstructure of tučekite resulting from the ordering in the cubic polyhedra of Sb and As (alternation of rectangles with different shades of grey in Fig. 5), which have very different mean bond-lengths (2.810 and 2.352 Å, respectively). We show here that the unit cell of arsenotučekite is multiple of the 7.2 Å × 5.4 Å tetragonal primitive cell of Just and Feather (1978) given for tučekite. Geometric relationships between the two cells are given in Fig. 6. The matrix to transform the cell of Just and Feather (1978) to the present one is |1–10/110/002|. It is very likely that tučekite also crystallizes in this space group.
The crystal structure of arsenotučekite down the a-axis. It can be considered as a superstructure of tučekite resulted from the ordering of Sb and As along the c-axis (alternation of rectangles with different shades of grey). Symbols as in Fig. 4. The unit cell and the orientation of the structure are outlined
The crystal structure of arsenotučekite down the c-axis. Geometric relationships with the tučekite unit cell (light-grey dashed square) are reported. Symbols as in Fig. 4. The unit cell and the orientation of the structure are outlined
Arsenotučekite does not correspond to any valid or invalid unnamed mineral (Smith and Nickel 2007). It is a new member of the hauchecornite group (Table 6), besides hauchecornite, bismutohauchecornite, tellurohauchecornite, arsenohauchecornite and tučekite (Gait and Harris 1980; Just 1980; Peacock 1950; Just and Feather 1978).
Origin of arsenotučekite
Based on previous and our mineralogical descriptions, it is possible to propose a genetic model for arsenotučekite. Arsenotučekite was not observed as inclusions in the magmatic chromite grains. Instead, it occurs as rims partly replacing pentlandite and as irregularly developed grains, locally associated with chlorite. These observations suggest that arsenotučekite likely precipitated at relatively low temperatures and two possible scenarios are proposed.
Arsenotučekite formed during (i) the late hydrothermal stages of the ore-forming process by reaction of Sb- and As-bearing solutions with magmatic sulfides such as pentlandite or (ii) during the serpentinization of the host peridotite. The first hypothesis was also proposed for tučekite described in the Kanowna nickel mineralization, its type locality, hosted in metamorphosed mafic and ultramafic rocks of the West Australian Archaean shield (Just and Feather 1978). The second hypothesis is in agreement with the model proposed by Economou and Naldrett (1984) for the formation of the interstitial sulfide described in the Tsangli chromitites. These authors suggested that the sulfides precipitated from hydrothermal fluids, which were also possibly responsible for the serpentinization of their host rocks. The source of the metals originates from the host rock.
References
Barth M, Gluhak T (2009) Geochemistry and tectonic setting of mafic rocks from the Othris Ophiolite, Greece. Contrib Mineral Petrol 157:23–40
Barth MG, Mason PRD, Davies GR, Dijkstra AH, Drury MR (2003) Geochemistry of the Othris ophiolite, Greece: evidence for refertilization? J Petrol 44:1759–1785
Barth MG, Mason PRD, Davies GR, Drury MR (2008) The Othris Ophiolite, Greece: a snapshot of subduction initiation at a mid-ocean ridge. Lithos 100:234–254
Bindi L, Zaccarini F, Ifandi E, Tsikouras B, Stanley C, Garuti G, Mauro D (2020a) Grammatikopoulosite, NiVP, a new phosphide from the chromitite of the Othrys Ophiolite, Greece. Minerals 10. https://doi.org/10.3390/min10020131
Bindi L, Zaccarini F, Bonazzi P, Grammatikoplous T, Tsikouras B, Stanley C, Garuti G (2020b) Eliopoulosite, V7S8, a new sulfide from the podiform chromitite of the Othrys Ophiolite, Greece. Minerals 10. https://doi.org/10.3390/min10030245
Bortolotti V, Chiari M, Marcucci M, Photiades A, Principi G, Saccani E (2008) New geochemical and age data on the ophiolites from the Othrys area (Greece): implication for the Triassic evolution of the Vardar Ocean. Ofioliti 33:135–151
Bruker (2016) APEX3, SAINT and SADABS. Bruker AXS Inc., Madison
Dijkstra AH, Barth MG, Drury MR, Mason PRD, Vissers RLM (2003) Diffuse porous melt flow and melt-rock reaction in the mantle lithosphere at a slow-spreading ridge: a structural petrology and LA-ICP-MS study of the Othris Peridotite Massif (Greece). Geochem Geophys Geosyst 4(8):8613
Economou MI, Naldrett AJ (1984) Sulfides associated with podiform bodies of chromite at Tsangli, Greece. Mineral Deposita 19:289–297
Gait RI, Harris DC (1980) Arsenohauchecornite and tellurohauchecornite: new minerals in the hauchecornite group. Mineral Mag 43:877–888
Grice GD, Ferguson RB (1989) The crystal structure of arsenohauchecornite. Can Mineral 27:137–142
Ifandi E, Zaccarini F, Tsikouras B, Grammatikopoulos T, Garuti G, Karipi S, Hatzipanagiotou K (2018) First occurrences of Ni-V-Co phosphides in chromitite of Agios Stefanos mine, Othrys ophiolite, Greece. Ofioliti 43:131–145
Just J (1980) Bismutohauchecornite - new name: hauchecornite redefined. Mineral Mag 43:873–876
Just J, Feather CE (1978) Tučekite, a new antimony analogue of hauchecornite. Mineral Mag 42(322):M21–M22
Koutsovitis P (2012) Gabbroic rocks in ophiolitic occurrences from East Othris, Greece: petrogenetic processes and geotectonic environment implications. Miner Petrol 104:249–265
Magganas A, Koutsovitis P (2015) Composition, melting and evolution of the upper mantle beneath the Jurassic Pindos ocean inferred by ophiolitic ultramafic rocks in east Othris, Greece. Int J Earth Sci 104:1185–1207
Peacock MA (1950) Hauchecornite. Am Mineral 35:440–446
Rassios A, Konstantopoulou G (1993) Emplacement tectonism and the position of chrome ores in the Mega Isoma peridotites, SW Othris, Greece. Bull Geol Soc Greece 28:463–474
Rassios A, Smith AG (2001) Constraints on the formation and emplacement age of western Greek ophiolites (Vourinos, Pindos, and Othris) inferred from deformation structures in peridotites. In: Dilek Y, Moores E, Elthon D, Nicolas A (eds) Ophiolites and oceanic crust: new insights from field studies and the ocean drilling program. Geol Soc Am Spec Pap, Boulder, pp 473–484
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A64:112–122
Smith DGW, Nickel EH (2007) A system for codification for unnamed minerals: report of the Subcommittee for Unnamed Minerals of the IMA Commission on new minerals, nomenclature and classification. Can Mineral 45:983–1055
Tsikouras B, Ifandi E, Karipi S, Grammatikopoulos TA, Hatzipanagiotou K (2016) Investigation of platinum-group minerals (PGM) from Othrys chromitites (Greece) using superpanning concentrates. Minerals 6. https://doi.org/10.3390/min6030094
Wilson AJC (ed) (1992) International tables for crystallography. Volume C: mathematical, physical and chemical tables. Kluwer Academic, Dordrecht
Zaccarini F, Bindi L, Ifandi E, Grammatikoplous T, Stanley C, Garuti G, Mauro D (2019) Tsikourasite, Mo3Ni2P1+x (x < 0.25), a new phosphide from the chromitite of the Othrys ophiolite, Greece. Minerals 9. https://doi.org/10.3390/min9040248
Acknowledgments
The authors acknowledge Ritsuro Miyawaki, Chairman of the Commission of New Minerals, Nomenclature and Classification (CNMNC), and commission members for helpful comments on the submitted new mineral proposal. We are grateful to the University Centre for Applied Geosciences (UCAG) for access to the Eugen F. Stumpfl Electron Microprobe Laboratory, which is operated by a consortium consisting of University of Leoben, Karl-Franzens University of Graz, and Graz University of Technology. SGS Mineral Services, Canada, is thanked for performing the concentrate sample. Funding was provided via Ministero dell’Università e della Ricerca project 2017AK8C32 ‘TEOREM deciphering geological processes using Terrestrial and Extraterrestrial ORE Minerals’ to L.B. We are grateful to two anonymous experts and handling editor Nikita V. Chukanov, whose comments helped to improve the manuscript.
Funding
Open access funding provided by Montanuniversität Leoben.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: N. V. Chukanov
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaccarini, F., Bindi, L., Tsikouras, B. et al. Arsenotučekite, Ni18Sb3AsS16, a new mineral from the Tsangli chromitites, Othrys ophiolite, Greece. Miner Petrol 114, 435–442 (2020). https://doi.org/10.1007/s00710-020-00712-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-020-00712-0