US20070287735A1 - Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases - Google Patents
Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases Download PDFInfo
- Publication number
- US20070287735A1 US20070287735A1 US11/785,998 US78599807A US2007287735A1 US 20070287735 A1 US20070287735 A1 US 20070287735A1 US 78599807 A US78599807 A US 78599807A US 2007287735 A1 US2007287735 A1 US 2007287735A1
- Authority
- US
- United States
- Prior art keywords
- group
- isoindol
- dioxo
- octahydro
- benzamide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 0 [1*]C([3*])C1C(=O)N(N([5*])C([6*])=O)C(=O)C1C([2*])[4*] Chemical compound [1*]C([3*])C1C(=O)N(N([5*])C([6*])=O)C(=O)C1C([2*])[4*] 0.000 description 10
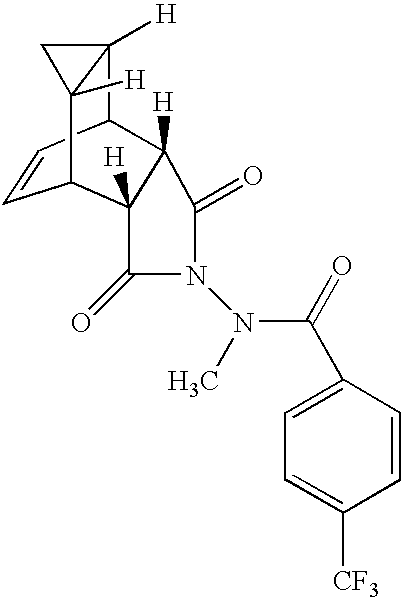
- CSKDFZIMJXRJGH-ADPPZMDZSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]12[H] CSKDFZIMJXRJGH-ADPPZMDZSA-N 0.000 description 2
- RWGFKTVRMDUZSP-UHFFFAOYSA-N CC(C)C1=CC=CC=C1 Chemical compound CC(C)C1=CC=CC=C1 RWGFKTVRMDUZSP-UHFFFAOYSA-N 0.000 description 1
- WATVQBOUPSCRIQ-KHOACFNVSA-N COc(cc1)ccc1C(NN(C([C@@H]1[C@H]2C3C=CC33C1C1C3C1)=O)C2=O)=O Chemical compound COc(cc1)ccc1C(NN(C([C@@H]1[C@H]2C3C=CC33C1C1C3C1)=O)C2=O)=O WATVQBOUPSCRIQ-KHOACFNVSA-N 0.000 description 1
- BBZORTDDQIPTKC-QDWHJOTQSA-N O=C(c(cc1)ccc1Br)NN(C([C@H]1[C@@H]2C(C3C4C3)C43C1CC3)=O)C2=O Chemical compound O=C(c(cc1)ccc1Br)NN(C([C@H]1[C@@H]2C(C3C4C3)C43C1CC3)=O)C2=O BBZORTDDQIPTKC-QDWHJOTQSA-N 0.000 description 1
- YIJMZTKVLKZOJS-QDWHJOTQSA-N O=C(c(cc1)ccc1F)NN(C([C@@H]1[C@H]2C3C=CC33C1C1C3C1)=O)C2=O Chemical compound O=C(c(cc1)ccc1F)NN(C([C@@H]1[C@H]2C3C=CC33C1C1C3C1)=O)C2=O YIJMZTKVLKZOJS-QDWHJOTQSA-N 0.000 description 1
- GARKLMBAQPJEGX-YCGFVIBYSA-N O=C(c1c[s]cc1)NN(C([C@@H]1[C@H]2C3C=CC33C1C1C3C1)=O)C2=O Chemical compound O=C(c1c[s]cc1)NN(C([C@@H]1[C@H]2C3C=CC33C1C1C3C1)=O)C2=O GARKLMBAQPJEGX-YCGFVIBYSA-N 0.000 description 1
- GNZBERNORFJFJT-CNHKKPJLSA-N [H]C12CC1([H])C1C(C)=C(C)C2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C(C)=C(C)C2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]12[H] GNZBERNORFJFJT-CNHKKPJLSA-N 0.000 description 1
- CSKDFZIMJXRJGH-UHFFFAOYSA-N [H]C12CC1([H])C1C=CC2C2([H])C(=O)N(NC(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)C12[H] Chemical compound [H]C12CC1([H])C1C=CC2C2([H])C(=O)N(NC(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)C12[H] CSKDFZIMJXRJGH-UHFFFAOYSA-N 0.000 description 1
- AZNCJZUWAHJCLB-YOHVKPHQSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(N(C)C(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(N(C)C(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]12[H] AZNCJZUWAHJCLB-YOHVKPHQSA-N 0.000 description 1
- PDTWJALCALXIIH-CNHKKPJLSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(N(CC)C(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(N(CC)C(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]12[H] PDTWJALCALXIIH-CNHKKPJLSA-N 0.000 description 1
- LHPDNQMEZBYLEE-UEZNURIYSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C34CC5CC(CC(C5)C3)C4)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C34CC5CC(CC(C5)C3)C4)C(=O)[C@]12[H] LHPDNQMEZBYLEE-UEZNURIYSA-N 0.000 description 1
- OLFJAAPWKAVFKS-GBMNLJDZSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=C(Br)C=CC=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=C(Br)C=CC=C3)C(=O)[C@]12[H] OLFJAAPWKAVFKS-GBMNLJDZSA-N 0.000 description 1
- CPRMSEKYJFJGII-AALLWRQTSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=C(C)N=C(C)S3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=C(C)N=C(C)S3)C(=O)[C@]12[H] CPRMSEKYJFJGII-AALLWRQTSA-N 0.000 description 1
- ATWGPAAGZWQRJW-GBMNLJDZSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=C(Cl)C=CC=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=C(Cl)C=CC=C3)C(=O)[C@]12[H] ATWGPAAGZWQRJW-GBMNLJDZSA-N 0.000 description 1
- IVYPVGFVVJXQCM-ADPPZMDZSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC(F)=CC=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC(F)=CC=C3)C(=O)[C@]12[H] IVYPVGFVVJXQCM-ADPPZMDZSA-N 0.000 description 1
- QRWZSMGXNJSJBA-ADPPZMDZSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(Br)C=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(Br)C=C3)C(=O)[C@]12[H] QRWZSMGXNJSJBA-ADPPZMDZSA-N 0.000 description 1
- XIDCFAYBJPWRRW-YOHVKPHQSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(C#N)C=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(C#N)C=C3)C(=O)[C@]12[H] XIDCFAYBJPWRRW-YOHVKPHQSA-N 0.000 description 1
- WRNZHKUBWSDTRF-YOHVKPHQSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(C)C=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(C)C=C3)C(=O)[C@]12[H] WRNZHKUBWSDTRF-YOHVKPHQSA-N 0.000 description 1
- USPKYYZDABFFSZ-ADPPZMDZSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(Cl)C=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(Cl)C=C3)C(=O)[C@]12[H] USPKYYZDABFFSZ-ADPPZMDZSA-N 0.000 description 1
- YIRDWOBUQUNSNY-ADPPZMDZSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(F)C=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(F)C=C3)C(=O)[C@]12[H] YIRDWOBUQUNSNY-ADPPZMDZSA-N 0.000 description 1
- ZPZCRHNAIUYXKE-YOHVKPHQSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(OC)C=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(OC)C=C3)C(=O)[C@]12[H] ZPZCRHNAIUYXKE-YOHVKPHQSA-N 0.000 description 1
- AGTXHHZRNMTLHL-ADPPZMDZSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C([N+](=O)[O-])C=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C([N+](=O)[O-])C=C3)C(=O)[C@]12[H] AGTXHHZRNMTLHL-ADPPZMDZSA-N 0.000 description 1
- IYWDNVOMIGEONH-ADPPZMDZSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=CC(Br)=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=CC(Br)=C3)C(=O)[C@]12[H] IYWDNVOMIGEONH-ADPPZMDZSA-N 0.000 description 1
- KVTCHDSYFPESLK-ADPPZMDZSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=CC(Cl)=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=CC(Cl)=C3)C(=O)[C@]12[H] KVTCHDSYFPESLK-ADPPZMDZSA-N 0.000 description 1
- PGAGSPWHPNHQCY-RLOIFVPSSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=CN=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=CN=C3)C(=O)[C@]12[H] PGAGSPWHPNHQCY-RLOIFVPSSA-N 0.000 description 1
- RGTBYNJLXMRUQN-RLOIFVPSSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=NC=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CC=NC=C3)C(=O)[C@]12[H] RGTBYNJLXMRUQN-RLOIFVPSSA-N 0.000 description 1
- GQGOBOCEBRFFFK-AALLWRQTSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CSC=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=CSC=C3)C(=O)[C@]12[H] GQGOBOCEBRFFFK-AALLWRQTSA-N 0.000 description 1
- UOHVZYYJHRCWBD-UHPCZEEKSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=NC=CC=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3=NC=CC=C3)C(=O)[C@]12[H] UOHVZYYJHRCWBD-UHPCZEEKSA-N 0.000 description 1
- DMQIKGOJSPZYLP-ADPPZMDZSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3CCCCC3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)C3CCCCC3)C(=O)[C@]12[H] DMQIKGOJSPZYLP-ADPPZMDZSA-N 0.000 description 1
- CVVKETMOOFDHOM-UMXUKVQPSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)CC3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)CC3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]12[H] CVVKETMOOFDHOM-UMXUKVQPSA-N 0.000 description 1
- MSJDEALLCYFPIJ-UMXUKVQPSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)CC3=CC=CC=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)CC3=CC=CC=C3)C(=O)[C@]12[H] MSJDEALLCYFPIJ-UMXUKVQPSA-N 0.000 description 1
- HGOZFVZTVSHXND-MGXKEQJRSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)CC3=CC=NC=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)CC3=CC=NC=C3)C(=O)[C@]12[H] HGOZFVZTVSHXND-MGXKEQJRSA-N 0.000 description 1
- OQPSLLKXDOIOQW-QRFKRNBUSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)CC=C)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(=O)CC=C)C(=O)[C@]12[H] OQPSLLKXDOIOQW-QRFKRNBUSA-N 0.000 description 1
- PVURAYFJHUXZCT-YZGMSDOFSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(C)=O)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)N(NC(C)=O)C(=O)[C@]12[H] PVURAYFJHUXZCT-YZGMSDOFSA-N 0.000 description 1
- QMLYJZNAGFCCGP-XIDJMXOJSA-N [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)OC(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1C=CC2[C@]2([H])C(=O)OC(=O)[C@]12[H] QMLYJZNAGFCCGP-XIDJMXOJSA-N 0.000 description 1
- NHMSINHXUXEFJT-ADPPZMDZSA-N [H]C12CC1([H])C1CCC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(Br)C=C3)C(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1CCC2[C@]2([H])C(=O)N(NC(=O)C3=CC=C(Br)C=C3)C(=O)[C@]12[H] NHMSINHXUXEFJT-ADPPZMDZSA-N 0.000 description 1
- NWWNOEMICJNZCT-XIDJMXOJSA-N [H]C12CC1([H])C1CCC2[C@]2([H])C(=O)OC(=O)[C@]12[H] Chemical compound [H]C12CC1([H])C1CCC2[C@]2([H])C(=O)OC(=O)[C@]12[H] NWWNOEMICJNZCT-XIDJMXOJSA-N 0.000 description 1
- KJDNIXCMYMBSNO-UHFFFAOYSA-N [H]N(C(=O)C1=CC=C(Br)C=C1)N1C(=O)C2CCCCC2C1=O Chemical compound [H]N(C(=O)C1=CC=C(Br)C=C1)N1C(=O)C2CCCCC2C1=O KJDNIXCMYMBSNO-UHFFFAOYSA-N 0.000 description 1
- HSZNSVLYPJLCRF-GHXBURCUSA-N [H][C@@]12C(=O)N(NC(=O)C3=C(Cl)C=C(Cl)C=C3)C(=O)[C@]1([H])C1C=CC2C1 Chemical compound [H][C@@]12C(=O)N(NC(=O)C3=C(Cl)C=C(Cl)C=C3)C(=O)[C@]1([H])C1C=CC2C1 HSZNSVLYPJLCRF-GHXBURCUSA-N 0.000 description 1
- RIMPTBZGDBXUFE-UZIHZSBWSA-N [H][C@@]12C(=O)N(NC(=O)C3=CC=C(Br)C=C3)C(=O)[C@]1([H])C1C=CC2C1 Chemical compound [H][C@@]12C(=O)N(NC(=O)C3=CC=C(Br)C=C3)C(=O)[C@]1([H])C1C=CC2C1 RIMPTBZGDBXUFE-UZIHZSBWSA-N 0.000 description 1
- CJAXQEDNHWIMQB-SUBVLCJKSA-N [H][C@@]12C(=O)N(NC(=O)C3=CC=C(Br)C=C3)C(=O)[C@]1([H])C1C=CC2CC1 Chemical compound [H][C@@]12C(=O)N(NC(=O)C3=CC=C(Br)C=C3)C(=O)[C@]1([H])C1C=CC2CC1 CJAXQEDNHWIMQB-SUBVLCJKSA-N 0.000 description 1
- KNUOUEDISNFRIY-JSHBZLHNSA-N [H][C@@]12C(=O)N(NC(=O)C3=CC=C(Br)C=C3)C(=O)[C@]1([H])C1C=CC2CCC1 Chemical compound [H][C@@]12C(=O)N(NC(=O)C3=CC=C(Br)C=C3)C(=O)[C@]1([H])C1C=CC2CCC1 KNUOUEDISNFRIY-JSHBZLHNSA-N 0.000 description 1
- LXMVJTNCOZWYQZ-SUBVLCJKSA-N [H][C@@]12C(=O)N(NC(=O)C3=CC=C(Br)C=C3)C(=O)[C@]1([H])C1CCC2CC1 Chemical compound [H][C@@]12C(=O)N(NC(=O)C3=CC=C(Br)C=C3)C(=O)[C@]1([H])C1CCC2CC1 LXMVJTNCOZWYQZ-SUBVLCJKSA-N 0.000 description 1
- RKZGYYBYKYLFHY-SUBVLCJKSA-N [H][C@@]12C(=O)N(NC(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]1([H])C1C=CC2C=C1 Chemical compound [H][C@@]12C(=O)N(NC(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]1([H])C1C=CC2C=C1 RKZGYYBYKYLFHY-SUBVLCJKSA-N 0.000 description 1
- BVOSYXIKTYKMPL-SUBVLCJKSA-N [H][C@@]12C(=O)N(NC(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]1([H])C1C=CC2CC1 Chemical compound [H][C@@]12C(=O)N(NC(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]1([H])C1C=CC2CC1 BVOSYXIKTYKMPL-SUBVLCJKSA-N 0.000 description 1
- QNBIPDPKATXWOJ-SUBVLCJKSA-N [H][C@@]12C(=O)N(NC(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]1([H])C1CCC2CC1 Chemical compound [H][C@@]12C(=O)N(NC(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@]1([H])C1CCC2CC1 QNBIPDPKATXWOJ-SUBVLCJKSA-N 0.000 description 1
- XOVTYEHPLKSKDZ-LLZFXZEUSA-N [H][C@@]12C(=O)N(NC(=O)C3=CC=CC(Br)=C3)C(=O)[C@]1([H])C1C=CC2C12CC2 Chemical compound [H][C@@]12C(=O)N(NC(=O)C3=CC=CC(Br)=C3)C(=O)[C@]1([H])C1C=CC2C12CC2 XOVTYEHPLKSKDZ-LLZFXZEUSA-N 0.000 description 1
- YIHKILSPWGDWPR-HYNHDVCUSA-N [H][C@@]12C(=O)OC(=O)[C@]1([H])C1C=CC2CC1 Chemical compound [H][C@@]12C(=O)OC(=O)[C@]1([H])C1C=CC2CC1 YIHKILSPWGDWPR-HYNHDVCUSA-N 0.000 description 1
- FGVZZSVCYPXXAC-WZENYGAOSA-N [H][C@@]12C(=O)OC(=O)[C@]1([H])C1C=CC2CCC1 Chemical compound [H][C@@]12C(=O)OC(=O)[C@]1([H])C1C=CC2CCC1 FGVZZSVCYPXXAC-WZENYGAOSA-N 0.000 description 1
- XOHZZDFJOIMTMY-KUAIZNHUSA-N [H][C@@]12C[C@]1([H])C1CCC2[C@@]2([H])C(=O)N(NC(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@@]12[H] Chemical compound [H][C@@]12C[C@]1([H])C1CCC2[C@@]2([H])C(=O)N(NC(=O)C3=CC=C(C(F)(F)F)C=C3)C(=O)[C@@]12[H] XOHZZDFJOIMTMY-KUAIZNHUSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/56—Ring systems containing three or more rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1617—Organic compounds, e.g. phospholipids, fats
- A61K9/1623—Sugars or sugar alcohols, e.g. lactose; Derivatives thereof; Homeopathic globules
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1652—Polysaccharides, e.g. alginate, cellulose derivatives; Cyclodextrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
Definitions
- Described herein are di, tri, and tetracyclic acylhydrazide derivatives and analogs, as well as compositions containing the same, for the treatment or prophylaxis of viral infections and diseases associated therewith, particularly those viral infections and associated diseases caused by the orthopoxvirus.
- the Orthopox genus (Orthopoxyiridae) is a member of the Poxyiridae family and the Chordopoxivirinae subfamily.
- the genus consists of numerous viruses that cause significant disease in human and animal populations. Viruses in the orthopox genus include cowpox, monkeypox, vaccinia, and variola (smallpox), all of which can infect humans.
- the smallpox (variola) virus is of particular importance. Recent concerns over the use of smallpox virus as a biological weapon have underscored the necessity of developing small molecule therapeutics that target orthopoxviruses. Variola virus is highly transmissible and causes severe disease in humans resulting in high mortality rates (Henderson et al. (1999) JAMA. 281:2127-2137). Moreover, there is precedent for use of variola virus as a biological weapon. During the French and Indian wars (1754-1765), British soldiers distributed blankets used by smallpox patients to American Indians in order to establish epidemics (Stern, E. W. and Stern, A. E. 1945. The effect of smallpox on the destiny of the Amerindian. Boston).
- Variola virus is naturally transmitted via aerosolized droplets to the respiratory mucosa where replication in lymph tissue produces asymptomatic infection that lasts 1-3 days. Virus is disseminated through the lymph to the skin where replication in the small dermal blood vessels and subsequent infection and lysis of adjacent epidermal cells produces skin lesions (Moss, B. (1990) Poxyiridae and Their Replication, 2079-2111. In B. N. Fields and D. M. Knipe (eds.), Fields Virology. Raven Press, Ltd., New York). Two forms of disease are associated with variola virus infection; variola major, the most common form of disease, which produces a 30% mortality rate and variola minor, which is less prevalent and rarely leads to death ( ⁇ 1%). Mortality is the result of disseminated intravascular coagulation, hypotension, and cardiovascular collapse, that can be exacerbated by clotting defects in the rare hemorrhagic type of smallpox (Moss, supra).
- monkeypox virus A recent outbreak of monkeypox virus underscores the need for developing small molecule therapeutics that target viruses in the orthopox genus. Appearance of monkeypox in the US represents an emerging infection. Monkeypox and smallpox cause similar diseases in humans, however mortality for monkeypox is lower (1%).
- Vaccination is the current means for preventing orthopox virus disease, particularly smallpox disease.
- the smallpox vaccine was developed using attenuated strains of vaccinia virus that replicate locally and provide protective immunity against variola virus in greater than 95% of vaccinated individuals (Modlin (2001) MMWR (Morb Mort Wkly Rep) 50:1-25).
- Adverse advents associated with vaccination occur frequently (1:5000) and include generalized vaccinia and inadvertent transfer of vaccinia from the vaccination site. More serious complications such as encephalitis occur at a rate of 1:300,000, which are often fatal (Modlin, supra). The risk of adverse events is even more pronounced in immunocompromised individuals (Engler et al.
- VIG Vaccinia virus immunoglobulin
- VIG is an isotonic sterile solution of immunoglobulin fraction of plasma derived from individuals who received the vaccinia virus vaccine. It is used to treat eczema vaccinatum and some forms of progressive vaccinia. Since this product is available in limited quantities and difficult to obtain, it has not been indicated for use in the event of a generalized smallpox outbreak (Modlin, supra).
- Cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine][HBMPC] is a nucleoside analog approved for treatment of CMV retinitis in AIDS patients. Cidofovir has been shown to have activity in vitro against a number of DNA containing viruses including adenovirus, herpesviruses, hepadnaviruses, polyomaviruses, papillomaviruses, and orthopoxviruses (Bronson et al. (1990) Adv. Exp. Med. Biol. 278:277-83; De Clercq et al. (1987) Antiviral Res.
- viruses including adenovirus, herpesviruses, hepadnaviruses, polyomaviruses, papillomaviruses, and orthopoxviruses
- Cidofovir has also been found to inhibit authentic variola virus replication (Smee et al. (2002) Antimicrob. Agents Chemother. 46:1329-1335).
- Cidofovir is poorly bioavailable and must be administered intravenously (Laezari et al. (1997) Ann. Intern. Med. 126:257-263). Moreover, cidofovir produces dose-limiting nephrotoxicity upon intravenous administration (Lalezari et al.). In addition, cidofovir-resistance has been noted for multiple viruses. Cidofovir-resistant cowpox, monkeypox, vaccinia, and camelpox virus variants have been isolated in the laboratory by repeated passage in the presence of drug (Smee, supra).
- Cidofovir-resistance represents a significant limitation for use of this compound to treat orthopoxvirus replication.
- the poor bioavailability, need for intravenous administration, and prevalence of resistant virus underscores the need for development of additional and alternative therapies to treat orthopoxvirus infection.
- methisazone the prototypical thiosemicarbazone
- methisazone has been used in the prophylactic treatment of smallpox infections (Bauer et al. (1969) Am. J Epidemiol. 90:130-145).
- this compound class has not garnered much attention since the eradication of smallpox due to generally unacceptable side effects such as severe nausea and vomiting.
- Mechanism of action studies suggest that methisazone interferes with translation of L genes (De Clercq (2001), supra).
- methisazone is a relatively non-specific antiviral compound and can inhibit a number of other viruses including adenoviruses, picornaviruses, reoviruses, arboviruses, and myxoviruses (Id.).
- SAH S-adenosylhomocysteine hydrolase
- orthopoxviruses including cowpox, monkeypox, camelpox, variola, and probably most other mammalian orthopoxviruses, can be grown readily in cell culture and produce robust cytopathic effect (CPE) in 3 to 5 days. Since this CPE is directly related to viral replication, compounds that inhibit virus replication in cell culture can be identified readily as conferring protection from virus-induced CPE (although it is theoretically possible to inhibit CPE without inhibiting virus replication). Moreover, compounds having identified activity against cowpox virus will also likely be active against human variola virus given the high degree of homology (>95%) between these two viruses and the fact that the replication proteins of orthopoxviruses are highly homologous.
- viruses diverge in regions of their genomes that encode immunomodulatory functions (host-specific). Additionally, many compounds have been identified in the literature that inhibit orthopoxvirus replication in cell culture and there are few, if any, examples where a compound is dramatically more potent against one species of orthopoxvirus and not the others
- Described herein are compounds and compositions and/or methods for the treatment and prophylaxis of viral infections, as well as diseases associated with viral infections in living hosts.
- the compounds described herein are of the following general formula:
- R 1 and R 2 represent radicals independently selected from the group consisting of hydrogen and alkyl
- R 3 and R 4 represent radicals independently selected from the group consisting of hydrogen and alkyl
- R 3 and R 4 taken together with the carbons to which they are attached form a cyclic structure selected from the group consisting of wherein R 7 , R 8 , R 9 , R 10 , R 11 , and R 12 represent radicals that are independently selected from the group consisting of hydrogen and alkyl;
- R 5 represents a radical selected from the group consisting of hydrogen and alkyl
- R 6 represents a radical selected from the group consisting of straight- or branched chain alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, cycloalkenyl, a substituted or unsubstituted aryl group, a substituted or unsubstituted heteroaryl group selected from the group consisting of furyl, thienyl, pyridyl, pyrrolyl, oxazolyl, thiazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, 1,2,3-oxadiazolyl, 1,2,3-triazolyl, and tetrazolyl; a substituted or unsubstituted arylalkyl group, and a substituted or unsubstituted heteroarylalkyl group, wherein the heteroaryl is selected from the group consisting pyridine and thiophene;
- M is selected from the group consisting of wherein R 13 , R 14 , R 15 , and R 16 are independently selected from the group consisting of hydrogen and alkyl;
- said aryl group substituents and said arylalkyl group substituents being one or more radical(s) independently selected from the group consisting of a straight- or branched chain alkyl, alkoxy, alkoxyalkyl, alkoxyalkoxy, halogen, polyfluoroalkyl, polyfluoroalkoxy, carboxy, cyano, nitro, amido, amidoalkyl, carboxamide, alkylthio, alkylsulfinyl, alkylsulfonyl, sulfonamide, and mercapto;
- heteroaryl group substituents and said heteroarylalkyl group substituents being one or more radical(s) independently selected from the group consisting of a straight- or branched chain alkyl, hydroxy, alkoxy, alkoxyalkyl, alkoxyalkoxy, halogen, polyfluoroalkyl, polyfluoroalkoxy, carboxy, cyano, amino, monoalkylamino, dialkylamino, aminoalkyl, nitro, amido, amidoalkyl, carboxamide, alkylthio, alkylsulfinyl, alkylsulfonyl, sulfonamide, and mercapto;
- compositions containing the antiviral compounds of Formula I are also described herein and the corresponding methods of use for treating and preventing infections caused by orthopox viruses.
- Compounds of Formula I include the compounds of Formula Ia: wherein:
- R 6 represents a radical selected from the group consisting of straight- or branched chain alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, cycloalkenyl, a substituted or unsubstituted aryl group, a substituted or unsubstituted heteroaryl group selected from the group consisting of furyl, thienyl, pyridyl, pyrrolyl, oxazolyl, thiazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, 1,2,3-oxadiazolyl, 1,2,3-triazolyl, and tetrazolyl; a substituted or unsubstituted arylalkyl group, and a substituted or unsubstituted heteroarylalkyl group, wherein the heteroaryl is selected from the group consisting pyridine and thiophene;
- said aryl group substituents and said arylalkyl group substituents being one or more radical(s) independently selected from the group consisting of a straight- or branched chain alkyl, alkoxy, alkoxyalkyl, alkoxyalkoxy, halogen, polyfluoroalkyl, polyfluoroalkoxy, carboxy, cyano, nitro, amido, amidoalkyl, carboxamide, alkylthio, alkylsulfinyl, alkylsulfonyl, sulfonamide, and mercapto;
- heteroaryl group substituents and said heteroarylalkyl group substituents being one or more radical(s) independently selected from the group consisting of a straight- or branched chain alkyl, hydroxy, alkoxy, alkoxyalkyl, alkoxyalkoxy, halogen, polyfluoroalkyl, polyfluoroalkoxy, carboxy, cyano, amino, monoalkylamino, dialkylamino, aminoalkyl, nitro, amido, amidoalkyl, carboxamide, alkylthio, alkylsulfinyl, alkylsulfonyl, sulfonamide, and mercapto;
- R 6 represents a radical selected from the group consisting of straight- or branched chain alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, cycloalkenyl, a substituted or unsubstituted aryl group, a substituted or unsubstituted heteroaryl group selected from the group consisting of furyl, thienyl, pyridyl, pyrrolyl, oxazolyl, thiazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, 1,2,3-oxadiazolyl, 1,2,3-triazolyl, and tetrazolyl; a substituted or unsubstituted arylalkyl group, and a substituted or unsubstituted heteroarylalkyl group, wherein the heteroaryl is selected from the group consisting pyridine and thiophene;
- said aryl group substituents and said arylalkyl group substituents being one or more radical(s) independently selected from the group consisting of a straight- or branched chain alkyl, alkoxy, alkoxyalkyl, alkoxyalkoxy, halogen, polyfluoroalkyl, polyfluoroalkoxy, carboxy, cyano, nitro, amido, amidoalkyl, carboxamide, alkylthio, alkylsulfinyl, alkylsulfonyl, sulfonamide, and mercapto;
- heteroaryl group substituents and said heteroarylalkyl group substituents being one or more radical(s) independently selected from the group consisting of a straight- or branched chain alkyl, hydroxy, alkoxy, alkoxyalkyl, alkoxyalkoxy, halogen, polyfluoroalkyl, polyfluoroalkoxy, carboxy, cyano, amino, monoalkylamino, dialkylamino, aminoalkyl, nitro, amido, amidoalkyl, carboxamide, alkylthio, alkylsulfinyl, alkylsulfonyl, sulfonamide, and mercapto;
- Exemplary compounds include 4-trifluoromethyl-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide; 4-bromo-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethanocycloprop[f]isoindol-2(1H)-yl)-benzamide; 4-bromo-N-(octahydro-1,3-dioxo-2H-isoindol-2-yl)-benzamide; 4-fluoro-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)
- the compound may be selected from any of the compounds described, supra.
- Also described herein is a method for preventing and treating orthopoxvirus infections and for preventing and treating diseases associated with such infections in a living host (for example, a mammal including a human) having or susceptible to an orthopoxvirus infection, comprising the step of administering to the living host a therapeutically effective amount of a compound of the formula:
- R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , and M are as defined for compounds of Formula I above, or a pharmaceutically acceptable salt to a host susceptible to, or suffering from such infection.
- Such methods include the prevention and treatment of orthopoxvirus infections and diseases associated with such infections in a living host having or susceptible to an orthopoxvirus infection, comprising the step of administering a therapeutically effective amount of the compounds of the Formula Ia, above, or a pharmaceutically acceptable salt thereof. Also described is the prophylaxis or treatment of orthopoxvirus infections and diseases associated with such infections in a living host having or susceptible to an orthopoxvirus infection, comprising the step of administering a therapeutically effective amount of the compounds of the Formula Ib, above or a pharmaceutically acceptable salt, thereof.
- orthopox virus is selected from the group consisting of vaccinia virus, cowpox virus, smallpox (variola) virus, monkeypox virus and camelpox virus; in a living host (for example, a mammal including a human) comprising the step of administering a therapeutically effective amount of the compounds of the invention to a host susceptible to, or suffering from such infection.
- compositions for the treatment or prevention of orthopoxvirus infections and diseases associated with such infections in a living host that comprises a therapeutically effective amount of one or more of the compounds of the formula:
- R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , and M are as defined for compounds of Formula I above, and a pharmaceutically acceptable carrier medium.
- the compounds described herein are particularly effective against orthopoxviruses, and are useful in the prophylaxis and/or treatment of infections and diseases associated with this virus in living hosts.
- orthopoxviruses examples include, but are not limited to, aractuba virus, BeAn 58058 virus, buffalopox virus, camelpox virus (such as Camelpox virus 903, Camelpox virus CMG, Camelpox virus CMS, Camelpox virus CP1, Camelpox virus CP5, and Camelpox virus M-96), cantagalo orthopoxvirus, cowpox virus (such as Cowpox virus strain Hamburg-1985 and Cowpox virus strain Turkmenia-1974), Ectromelia virus (such as Belo Horizonte virus), elephantpox virus, monkeypox virus (such as Monkeypox virus strain Sierra Leone 70-0266 and Monkeypox virus strain Zaire 77-0666), rabbitpox virus (such as Rabbitpox strain Utrecht), raccoonpox virus, skunkpox virus, taterapox virus, vaccinia
- antiviral activity of representative compounds was evaluated in assays that measure the ability of compounds to protect cells from virus-induced CPE.
- Cells that will support growth of the particular orthopox virus strain are seeded into 96-well tissue culture treated plates and then infected with an amount of the appropriate orthopox virus strain that results in complete CPE in ⁇ 3 days.
- Various dilutions of inhibitory compound(s) are added and the plates are incubated at the appropriate temperature for optimal virus growth. At the end of the incubation period, cells are fixed with glutaraldehyde and stained with crystal violet.
- Cell protection is measured spectrophotometrically at OD 570 nm.
- the interpolated compound dilution that results in 50% protection of the cell monolayer from virus-induced CPE is calculated and reported as the 50% effective concentration or EC 50 .
- Antiviral activity of representative compounds described herein occurred at drug concentrations that had no demonstrable effect on cell growth, indicating that the compounds were working specifically by an antiviral mechanism.
- the compounds described herein collectively, include the compounds of Formula I, pharmaceutically acceptable salts thereof, their isomers, and mixtures thereof.
- the compounds are identified herein by their chemical structure and/or chemical name. Where a compound is referred to by both a chemical structure and a chemical name, and that chemical structure and chemical name conflict, the chemical structure is determinative of the compound's identity.
- living host refers to an organism that is living and capable of being infected with a virus, such as an orthopoxvirus; for example, a mammal, which includes a human.
- a virus such as an orthopoxvirus
- alkyl refers to straight or branched chain aliphatic hydrocarbon radicals of up to 10 carbon atoms, preferably up to 6 carbon atoms and more preferably 1 to 4 carbon atoms.
- alkoxy —O-alkyl
- allylthio —S-alkyl
- monoalkylamino —NH-alkyl
- dialkylamino —N-(alkyl)alkyl
- alkylsulfonyl —S(O) 2 -alkyl
- carboxyalkyl -alkyl-COOH
- substituents such as alkoxy (—O-alkyl), allylthio (—S-alkyl), monoalkylamino (—NH-alkyl), dialkylamino (—N-(alkyl)alkyl), alkylsulfonyl (—S(O) 2 -alkyl), carboxyalkyl (-alkyl-COOH), or the like, also refers
- alk in structural formula denotes an alkyl group, unless divalency is indicated in which case the “alk” denotes the corresponding alkylene group(s). Additionally, the term “lower alkyl” denotes an alkyl group having one to four carbon atoms.
- alkenyl refers to straight or branched chain aliphatic hydrocarbon radicals of 2 to 7 carbon atoms containing one double bond. Such alkenyl moieties may exist in the E or Z configurations; the compounds of this invention include both configurations.
- alkynyl refers to straight or branched chain aliphatic hydrocarbon radicals containing 2 to 7 carbon atoms having at least one triple bond.
- phenyl refers to a group.
- substituted phenyl refers to a phenyl group that is substituted with the indicated substituents.
- aryl when used as such, refers to an aromatic carbocyclic group, having 6 to 10 carbon atoms including without limitation phenyl and napthyl.
- heteroaryl refers to a 5- or 6-membered aromatic cyclic group having at least one carbon atom and one or more oxygen, nitrogen or sulfur atoms in the ring, as for example furyl, thienyl, pyridyl, pyrrolyl, oxazolyl, thiazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, 1,2,3-oxadiazolyl, 1,2,3-triazolyl, tetrazolyl, and the like, including all position isomers.
- Preferred heteroaryl groups include pyridine, thiazole and thiophene.
- cycloalkyl refers to a saturated hydrocarbon ring. Cycloalkyls can be monocyclic or can be fused, spiro or bridged bicyclic or tricyclic ring systems. Monocyclic cycloalkyl rings contain from 3 to 10 carbon atoms, preferably from 3 to 7 carbon atoms, as for example cyclopropyl, cyclobutyl, cyclopentyl, and cyclohexyl.
- Bicyclic and tricyclic cycloalkyl rings contain from 7 to 28 carbon atoms, preferably from 7 to 19 carbon atoms, in the ring system; and include, for example, adamantyl, bicyclo[2.2.1]heptyl, bicyclo[2.2.2]cyclooctanyl, tricyclo[3.2.2.02,4]nonyl and norbornyl, and bicyclo[3.2.2]nonyl.
- cycloalkenyl refers to an unsaturated hydrocarbon ring. Cycloalkenyl rings are non-aromatic and contain at least one (preferably only one) carbon-carbon double bond.
- Cycloalkenyl rings are monocyclic, or are fused, spiro or bridged bicyclic or tricyclic ring systems.
- Monocyclic cycloalkenyl rings contain from 5 to 10 carbon atoms, preferably from 5 to 7 carbon atoms, and include, for example, cyclopropenyl, cyclobutenyl, cyclopentenyl, and cyclohexenyl.
- Bicyclic and tricyclic cycloalkenyl rings contain from 7 to 28 carbon atoms in the ring, preferably from 7 to 19 carbon atoms, in the ring system; and include, for example, bicyclo[2.2.1]hept-2-ene, bicyclo[2.2.2]cyclooct-2-enyl, tricyclo[3.2.2.02,4]non-6-enyl, and bicyclo[3.2.2]non-6-enyl.
- carboxylate refers to a radical or substituent of the formula —C( ⁇ O)—NR′′R′′′, wherein R′′ and R′′′ are as previously defined.
- sulfonamide refers to a radical or substituent of the formula —SO 2 NR′′R′′′ or —NR′′SO 2 R′′′, wherein R′′ and R′′′ are as previously defined.
- halogen refers to a radical or substituent selected from the group consisting of chloro, bromo, iodo, and fluoro.
- HPLC refers to high-performance liquid chromatography.
- “Substituted” is intended to indicate that one or more hydrogens on the atom indicated in the expression using “substituted” is replaced with a selection from the indicated group(s), provided that the indicated atom's normal valency is not exceeded, and that the substitution results in a stable compound: When a substituent is an oxo ( ⁇ O) group, then 2 hydrogens on the atom are replaced.
- the compounds described herein and their pharmaceutically acceptable salts are useful in treating and preventing viral infections and diseases in living hosts when used in combination with other active agents, including but not limited to interferons, ribavirin, immunoglobulins, immunomodulators, anti-inflammatory agents, antibiotics, antivirals, anti-infectious agents, and the like.
- Compounds described herein are also useful in preventing or resolving orthopox viral infections in cell, tissue or organ cultures and other in vitro applications.
- inclusion of compounds of the invention as a supplement in cell or tissue culture growth media and cell or tissue culture components will prevent viral infections or contaminations of cultures not previously infected with viruses.
- Compounds described above may also be used to eliminate or attenuate viral replication in cultures or other biological materials infected or contaminated with viruses (for example, blood), after a suitable treatment period, under any number of treatment conditions as determined by the skilled artisan.
- the compounds described herein can form useful salts with inorganic and organic acids such as hydrochloric, sulfuric, acetic, lactic, or the like and with inorganic or organic bases such as sodium or potassium hydroxide, piperidine, ammonium hydroxide, or the like.
- inorganic or organic bases such as sodium or potassium hydroxide, piperidine, ammonium hydroxide, or the like.
- the pharmaceutically acceptable salts of the compounds of Formula I are prepared following procedures that are familiar to those skilled in the art.
- phrases “pharmaceutically acceptable” is employed herein to refer to those compounds, materials, compositions, and/or dosage forms which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of human beings and animals without excessive toxicity, irritation, allergic response, or other problem or complication commensurate with a reasonable benefit/risk ratio.
- the compounds described herein may have at least one chiral center, the compounds may thus exist as enantiomers.
- the compounds described herein may also possess two or more chiral centers and thus may also exist as diastereomers or as exo or endo isomers.
- these isomers may be separated by conventional techniques such as preparative chromatography. Accordingly, the compounds may be prepared as a racemic mixture or, by either enantiospecific synthesis or resolution, as individual enantiomers.
- the compounds may, for example, be resolved from a racemic mixture into their component racemates by standard techniques, such as the formation of diastereomeric pairs by salt formation with an optically active acid, such as ( ⁇ )-di-p-toluoyl-d-tartaric acid and/or (+)-di-p-toluoyl-1-tartaric acid followed by fractional crystallization and regeneration of the free base.
- the racemic mixture may also be resolved by formation of diastereomeric esters or amides, followed by chromatographic separation and removal of the chiral auxiliary.
- the compounds may be resolved using a chiral HPLC column. It is to be understood that all such isomers and mixtures thereof are encompassed within the scope described herein.
- the compounds described herein are useful for treating orthopoxvirus infection in living hosts, for example, mammals including humans. When administered to a living host the compounds can be used alone, or as a pharmaceutical composition.
- compositions comprising the compounds described herein, either alone or in combination with each other, offer a treatment against orthopoxvirus infection.
- the antiviral pharmaceutical compositions described herein comprise one or more of the compound(s) of Formula I above, as the active ingredient in combination with a pharmaceutically acceptable carrier medium or auxiliary agent.
- composition may be prepared in various forms for administration, including tablets, caplets, pills or dragees, or can be filled in suitable containers, such as capsules, or, in the case of suspensions, filled into bottles.
- pharmaceutically acceptable carrier medium includes any and all solvents, diluents, or other liquid vehicle, dispersion or suspension aids, surface active agents, isotonic agents, thickening or emulsifying agents, preservatives, solid binders, lubricants and the like, as suited to the particular dosage form desired.
- Gennaro (William and Wilkins, Baltimore, Md., 2000) discloses various carriers used in formulating pharmaceutical compositions and known techniques for the preparation thereof. Except insofar as any conventional carrier medium is incompatible with the antiviral compounds of the invention, such as by producing any undesirable biological effect or otherwise interacting in a deleterious manner with any other component(s) of the pharmaceutical composition, its use is contemplated to be within the scope of the compositions described herein.
- a pharmaceutical composition may include one or more water-insoluble diluents, water-soluble diluents, disintegrants, binders, wetting agents, solubilizers, glidants, lubricants, granulating solvents.
- An example of a suitable water-insoluble diluent is microcrystalline cellulose.
- An example of a suitable water-soluble diluent is lactose monohydrate.
- An example of a suitable typical disintegrant is croscarmellose sodium.
- An example of a suitable binder is hydroxypropylmethyl cellulose.
- An example of a suitable glidant is colloidal silicone dioxide.
- An example of a suitable lubricant is magnesium stearate.
- An example of a suitable granulating solvent is water.
- the active agent may be present in an amount of at least 0.5% and generally not more than 90% by weight, based on the total weight of the composition, including carrier medium and/or auxiliary agent(s), if any.
- the proportion of active agent varies between 5 to 50% by weight of the composition.
- compositions suitable for enteral or parenteral administration can be used to make up the composition.
- Gelatine, lactose, starch, magnesium stearate, talc, vegetable and animal fats and oils, gum, polyalkylene glycol, or other known medicament components may all be suitable as carrier media or excipients.
- the compounds described herein may be administered using any amount and any route of administration effective for attenuating infectivity of the virus.
- amount effective to attenuate infectivity of virus refers to a nontoxic but sufficient amount of the antiviral agent to provide the desired prophylaxis and/or treatment of viral infection. The exact amount required will vary from subject to subject, depending on the species, age, and general condition of the subject, the severity of the infection, the particular antiviral agent, its mode of administration, and the like.
- the compounds described herein may be administered within 24 hours of symptom onset, although therapeutic effects may be produced with administration within 48 hours of symptom onset, or even within 72 hours of symptom onset.
- Symptoms of initial orthopoxvirus infections depend on the exact virus contracted. For example, the initial symptoms of a smallpox infection include fever, malaise, head and body aches, and sometimes vomiting.
- the antiviral compounds may be formulated in dosage unit form for ease of administration and uniformity of dosage.
- dosage unit form refers to a physically discrete unit of antiviral agent appropriate for the patient to be treated.
- Each dosage should contain the quantity of active material calculated to produce the desired therapeutic effect either as such, or in association with the selected pharmaceutical carrier medium and/or the supplemental active agent(s), if any.
- the antiviral compounds of the invention will be administered in dosage units containing from about 100 mg to about 2,000 mg of the antiviral agent by weight of the composition, although dosage units containing from about 10 mg up to about 10,000 mg may also be employed.
- the compounds may be administered orally, rectally, parenterally, such as by intramuscular injection, subcutaneous injection, intravenous infusion or the like, intracisternally, intravaginally, intraperitoneally, locally, such as by powders, ointments, or drops, or the like, or by inhalation, such as by aerosol or the like, taking into account the nature and severity of the infection being treated.
- the compounds of the invention may be administered at dosage levels of about 0.125 to about 250 mg/kg of subject body weight per dose, one or more times a day, to obtain the desired therapeutic effect.
- the compounds of the invention will typically be administered from 1 to 4 times a day so as to deliver the above-mentioned daily dosage.
- the exact regimen for administration of the compounds and compositions described herein will necessarily be dependent on the needs of the individual host or patient being treated, the type of treatment administered and the judgment of the attending medical specialist.
- compounds of the invention may be administered within 48 hours after possible exposure, although effective prophylaxis can be produced by administration within 7 days post exposure, up to as long as 14 days post exposure.
- the dosages may be essentially the same, whether for treatment or prophylaxis of virus infection.
- any of the processes for preparation of the compounds described herein it may be necessary and/or desirable to protect sensitive or reactive groups on any of the molecules concerned. This may be achieved by means of conventional protecting groups, such as those described in Protective Groups in Organic Chemistry, ed. J. F. W. McOmie, Plenum Press, 1973; and T. W. Greene & P. G. M. Wuts, Protective Groups in Organic Synthesis, John Wiley & Sons, 1999.
- the protecting groups may be removed at a convenient subsequent stage using methods known from the art.
- Example 2-14 The compounds of Examples 2-14 were synthesized following the above mentioned general procedure for Example 1 using compound I (a) and reacting it with the following hydrazides: isonicotinic hydrazide, 4-bromobenzoic hydrazide, 3-bromobenzoic hydrazide, 3-chlorobenzoic hydrazide, 2-bromobenzoic hydrazide, 2-chlorobenzoic hydrazide, 4-chlorobenzoic hydrazide, nicotinic hydrazide, 2-picolinyl hydrazide, 4-methoxybenzoic hydrazide, 4-nitrobenzoic hydrazide, 4-fluorobenzoic hydrazide, and 3-fluorobenzoic hydrazide.
- hydrazides isonicotinic hydrazide, 4-bromobenzoic hydrazide, 3-bromobenzoic hydrazide, 3-chlorobenzo
- Virus stocks of Vaccinia virus were prepared in Vero cells infected at low multiplicity (0.01 plaque forming units (PFU)/cell) and harvested when cytopathic effects were complete (4+CPE). The samples were frozen and thawed and then sonicated to release cell-associated virus. The cell debris was removed by low-speed centrifugation, and the resulting virus suspension was stored in 1 mL aliquots at ⁇ 80° C. The PFU/mL of the virus suspension was quantified by standard plaque assay on Vero and BSC-40 cells.
- PFU plaque forming units
- Vero cell monolayers were seeded on to 96-well plates and infected with 2-fold serial dilutions of the vaccinia virus stock. At 3 days post-infection, the cultures were fixed with 5% glutaraldehyde and stained with 0.1% crystal violet. Virus-induced CPE was quantified spectrophotometrically at OD 570 . From this analysis, a 1:800 dilution of vaccinia virus stock was chosen for use in the HTS assay. This amount of vaccinia virus represents a multiplicity of infection of approximately 0.1 PFU/cell.
- Vero cell monolayers were infected with 1:800 dilution of vaccinia virus stock.
- Each plate contained the following controls: quadruplicate virus-infected wells, quadruplicate uninfected cell wells and a dose response curve in duplicate for cidofovir (CDV) added at 300, 100, 30 and 10 DAM, or phosphonoacetic acid (PAA) added at 2100, 714, 210, and 71. ⁇ M as reference standards.
- CDV cidofovir
- PAA phosphonoacetic acid
- the S/N ratio ratio of signal of cell control wells (signal) to virus control wells (noise)
- the well-to-well and assay-to-assay variability was less than 20%.
- the EC 50 values for CDV and PAA were determined to be 84 ⁇ 15 ⁇ M and 985 ⁇ 85 ⁇ M, respectively. These values were within the range of published values for these compounds. Based on this analysis, the 1:800 dilution of vaccinia virus (boxed) was chosen for use in the assay.
- Representative compounds of the invention were tested in the vaccinia virus CPE assay. Compounds were dissolved in DMSO and diluted in medium such that the final concentration in each well was 5 ⁇ M compound and 0.5% DMSO. The compounds were added robotically to the culture medium using the Biomek.RTM. FX robot system. Following compound addition, the cultures were infected with vaccinia virus. After 3 days, plates were processed and CPE quantified as described.
- Representative compounds of the invention inhibited vaccinia virus-induced CPE by great than 50% at the test concentration (5 ⁇ M). Selected compounds were further evaluated for potency (EC 50 ) in the CPE assay and cytotoxicity (CC 50 ) in an MTT assay.
- the MTT assay measures mitochondrial dehydrogenase activity in dividing cells. This method detects the in situ reduction of (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl-)-2H-tetrazolium) using an electron coupling reagent (phenazine methosulfate) to produce an insoluble formazan.
- the absorbance of the formazan at 490 nm can be measured directly from 96-well assay plates following solubilization of the formazan in 50% ethanol. The quantity of formazan product is directly proportional to the number of living cells in culture.
- EC 50 values are determined by comparing compound-treated and compound-untreated cells using a computer program. (The EC 50 value measures compound concentration that inhibits viral replication by 50%). The EC 50 values of representative compounds of the invention in the CPE assay are listed in Table 3, below. These compounds were active at non-toxic concentrations.
- Table 4 lists EC 50 values of select compounds of the invention measuring anti-orthopox virus activities in these CPE inhibition assays for cidofovir-resistant cowpox virus (Brighton Red strain, (available from USAMRIID Fort Detrick, Frederick, Md.), camelpox, and monkeypox virus (Zaire(V79-1-005-scab)).
- ST-246 is a white to off-white powder.
- the calculated log P is 2.94. Based on the partition coefficient, ST-246 is expected to have good permeability.
- the drug substance is micronized to improve its dissolution in the gastrointestinal fluids.
- the typical particle size of the micronized material is 50% less than 5 microns.
- Solubility The solubility of ST-246 is low in water (0.026 mg/mL) and buffers of the gastric pH range. Surfactant increases its solubility slightly. ST-246 is very soluble in organic solvents. The solubility data are given in Table 5. TABLE 5 Solubility of ST-246 in Various Diluents Diluent ST-246 Solubility (mg/mL) Phosphate Buffer, pH 6.8 0.00700 Water 0.0261 0.01N HCl 0.0375 1% Sodium Laurel Sulfate 0.0687 1% Tween 80 ® 0.102 Methanol 60.8 Ethanol 62.1 Acetonitrile 64.0
- Polymorphism X-ray diffraction pattern indicated crystalline material and is similar for various batches. DSC shows sharp endotherms at 196° C. corresponding to the melting point. There are small endotherms between 115° C. and 189° C. The position of these endotherms varies in the two batches.
- Biopharmaceutical Classification ST-246 is classified as BCS Class 2 due to its low solubility in solutions of gastric pH range and good permeability.
- micronized ST-246 GMP batch 06-0503 16-04/04-24-0 1 (Batch # Micronized 5C020) intended for clinical trial was placed on a three-year stability program in the current container (amber glass bottles) according to International Conference for Harmonization (ICH) guidelines. Six-month data indicate that ST-246 is stable at 25° C./60% RH and 40° C./75% RH and light.
- ST-246 may be manufactured in a process comprising the following four steps:
- Step 1 A solution of cycloheptatriene (1) and maleic anhydride (2) in anhydrous toluene is heated at 80° for 4 hours under a nitrogen atmosphere. After GC/MS analysis shows the reactions is complete, the reaction solution is cooled to room temperature and evaporated under reduced pressure. The resulting residue is recrystallized from tert-butyl methyl ether to afford the endo-isomer (3) as a white crystal.
- Step 2 To a solution of anhydrous hydrazine in anhydrous toluene is added methyl 4-(trifluoromethyl)benzoate (4). The reaction solution is heated at reflux for 18 hours under a nitrogen atmosphere. After cooling to 40-50° C., the solvent is evaporated under reduced pressure. The resulting solid is recrystallized from tert-butyl methyl ether to give hydrazide (5) as a white solid.
- Step 3 A mixture of the endo-isomer (3), the hydrazide (5), and ethanol is heated at reflux for 18 hours under a nitrogen atmosphere. The resulting solution is cooled to room temperature and concentrated in vacuo. The crude material (6) is used directly for the next step.
- Step 4 The crude material (6) is recrystallized from ethyl acetate and hexanes to obtain ST-246 (7) as a white solid. The material is dried for 48 hours at 40° C.
- the material may be packaged in amber glass bottles and stored at 2 to 8° C.
- ST-246 The physical form for ST-246 is a white to off-white solid.
- the molecular formula is C 19 H 15 F 3 N 2 O 3 .
- the molecular weight is 376.33.
- the melting point is 196° C. by DSC.
- the solubility of ST-246 is low in water (0.026 mg/mL) and slightly in buffers of the gastric pH range.
- ST-246 is very soluble in organic solvents (60 mg/mL).
- ST-246 can be formulated for oral administration in, for example, size 0 capsules containing either 25 mg or 200 mg ST-246. All inactive ingredients may be GRAS and USPLNF excipients.
- the manufacturing process may include wet granulation using a high shear mixer/granulator and filling into hard-gelatin capsules.
- Microcrystalline cellulose, NF (Avicel PH 101)
- Suitable dosage forms include capsules containing various amounts of active ingredient.
- the quantitative composition of two exemplary dosage forms containing 25 or 200 mg of ST-246 are listed below in Table 6.
- TABLE 6 Quantitative Composition for ST-246 Drug Product 200 mg strength 25 mg strength
Abstract
Description
- This application is a Continuation-in-Part of U.S. application Ser. No. 10/561,153, filed Apr. 5, 2006, which claims priority to International Patent Application Serial No. PCT/2004/112718, filed Jun. 18, 2004, which claims priority to U.S. Provisional Application No. 60/480,182, filed Jun. 20, 2003. All priority applications in their entireties are incorporated by reference herein for all purposes.
- Described herein are di, tri, and tetracyclic acylhydrazide derivatives and analogs, as well as compositions containing the same, for the treatment or prophylaxis of viral infections and diseases associated therewith, particularly those viral infections and associated diseases caused by the orthopoxvirus.
- The Orthopox genus (Orthopoxyiridae) is a member of the Poxyiridae family and the Chordopoxivirinae subfamily. The genus consists of numerous viruses that cause significant disease in human and animal populations. Viruses in the orthopox genus include cowpox, monkeypox, vaccinia, and variola (smallpox), all of which can infect humans.
- The smallpox (variola) virus is of particular importance. Recent concerns over the use of smallpox virus as a biological weapon have underscored the necessity of developing small molecule therapeutics that target orthopoxviruses. Variola virus is highly transmissible and causes severe disease in humans resulting in high mortality rates (Henderson et al. (1999) JAMA. 281:2127-2137). Moreover, there is precedent for use of variola virus as a biological weapon. During the French and Indian wars (1754-1765), British soldiers distributed blankets used by smallpox patients to American Indians in order to establish epidemics (Stern, E. W. and Stern, A. E. 1945. The effect of smallpox on the destiny of the Amerindian. Boston). The resulting outbreaks caused 50% mortality in some Indian tribes (Stern, E. W. and Stern, A. E.). More recently, the Soviet government launched a program to produce highly virulent weaponized forms of variola in aerosolized suspensions (Henderson, supra). Of more concern is the observation that recombinant forms of poxvirus have been developed that have the potential of causing disease in vaccinated animals (Jackson et al. (2001) J. Virol., 75:1205-1210).
- The smallpox vaccine program was terminated in 1972; thus, many individuals are no longer immune to smallpox infection. Even vaccinated individuals may no longer be fully protected, especially against highly virulent or recombinant strains of virus (Downie and McCarthy. (1958) J Hyg. 56:479-487; Jackson, supra). Therefore, mortality rates would be high if variola virus were reintroduced into the human population either deliberately or accidentally.
- Variola virus is naturally transmitted via aerosolized droplets to the respiratory mucosa where replication in lymph tissue produces asymptomatic infection that lasts 1-3 days. Virus is disseminated through the lymph to the skin where replication in the small dermal blood vessels and subsequent infection and lysis of adjacent epidermal cells produces skin lesions (Moss, B. (1990) Poxyiridae and Their Replication, 2079-2111. In B. N. Fields and D. M. Knipe (eds.), Fields Virology. Raven Press, Ltd., New York). Two forms of disease are associated with variola virus infection; variola major, the most common form of disease, which produces a 30% mortality rate and variola minor, which is less prevalent and rarely leads to death (<1%). Mortality is the result of disseminated intravascular coagulation, hypotension, and cardiovascular collapse, that can be exacerbated by clotting defects in the rare hemorrhagic type of smallpox (Moss, supra).
- A recent outbreak of monkeypox virus underscores the need for developing small molecule therapeutics that target viruses in the orthopox genus. Appearance of monkeypox in the US represents an emerging infection. Monkeypox and smallpox cause similar diseases in humans, however mortality for monkeypox is lower (1%).
- Vaccination is the current means for preventing orthopox virus disease, particularly smallpox disease. The smallpox vaccine was developed using attenuated strains of vaccinia virus that replicate locally and provide protective immunity against variola virus in greater than 95% of vaccinated individuals (Modlin (2001) MMWR (Morb Mort Wkly Rep) 50:1-25). Adverse advents associated with vaccination occur frequently (1:5000) and include generalized vaccinia and inadvertent transfer of vaccinia from the vaccination site. More serious complications such as encephalitis occur at a rate of 1:300,000, which are often fatal (Modlin, supra). The risk of adverse events is even more pronounced in immunocompromised individuals (Engler et al. (2002) J Allergy Clin Immunol. 110:357-365). Thus, vaccination is contraindicated for people with AIDS or allergic skin diseases (Engler et al.). While protective immunity lasts for many years, the antibody response to smallpox vaccination is significantly reduced 10 to 15 years post inoculation (Downie, supra). In addition, vaccination may not be protective against recombinant forms of orthopoxvirus. A recent study showed that recombinant forms of mousepox virus that express IL-4 cause death in vaccinated mice (Jackson, supra). Given the side effects associated with vaccination, contraindication of immunocompromised individuals, and inability to protect against recombinant strains of virus, better preventatives and/or new therapeutics for treatment of smallpox virus infection are needed.
- Vaccinia virus immunoglobulin (VIG) has been used for the treatment of post-vaccination complications. VIG is an isotonic sterile solution of immunoglobulin fraction of plasma derived from individuals who received the vaccinia virus vaccine. It is used to treat eczema vaccinatum and some forms of progressive vaccinia. Since this product is available in limited quantities and difficult to obtain, it has not been indicated for use in the event of a generalized smallpox outbreak (Modlin, supra).
- Cidofovir ([(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine][HBMPC]) is a nucleoside analog approved for treatment of CMV retinitis in AIDS patients. Cidofovir has been shown to have activity in vitro against a number of DNA containing viruses including adenovirus, herpesviruses, hepadnaviruses, polyomaviruses, papillomaviruses, and orthopoxviruses (Bronson et al. (1990) Adv. Exp. Med. Biol. 278:277-83; De Clercq et al. (1987) Antiviral Res. 8:261-272; de Oliveira et al. (1996) Antiviral Res. 31:165-172; Snoeck et al. (2001) Clin Infect. Dis. 33:597-602). Cidofovir has also been found to inhibit authentic variola virus replication (Smee et al. (2002) Antimicrob. Agents Chemother. 46:1329-1335).
- However, cidofovir administration is associated with a number of issues. Cidofovir is poorly bioavailable and must be administered intravenously (Laezari et al. (1997) Ann. Intern. Med. 126:257-263). Moreover, cidofovir produces dose-limiting nephrotoxicity upon intravenous administration (Lalezari et al.). In addition, cidofovir-resistance has been noted for multiple viruses. Cidofovir-resistant cowpox, monkeypox, vaccinia, and camelpox virus variants have been isolated in the laboratory by repeated passage in the presence of drug (Smee, supra). Cidofovir-resistance represents a significant limitation for use of this compound to treat orthopoxvirus replication. Thus, the poor bioavailability, need for intravenous administration, and prevalence of resistant virus underscores the need for development of additional and alternative therapies to treat orthopoxvirus infection.
- In addition to viral polymerase inhibitors such as cidofovir, a number of other compounds have been reported to inhibit orthopoxvirus replication (De Clercq. (2001) Clin Microbiol. Rev. 14:382-397). Historically, methisazone, the prototypical thiosemicarbazone, has been used in the prophylactic treatment of smallpox infections (Bauer et al. (1969) Am. J Epidemiol. 90:130-145). However, this compound class has not garnered much attention since the eradication of smallpox due to generally unacceptable side effects such as severe nausea and vomiting. Mechanism of action studies suggest that methisazone interferes with translation of L genes (De Clercq (2001), supra). Like cidofovir, methisazone is a relatively non-specific antiviral compound and can inhibit a number of other viruses including adenoviruses, picornaviruses, reoviruses, arboviruses, and myxoviruses (Id.).
- Another class of compounds potentially useful for the treatment of poxviruses is represented by inhibitors of S-adenosylhomocysteine hydrolase (SAH). This enzyme is responsible for the conversion of S-adenosylhomocysteine to adenosine and homocysteine, a necessary step in the methylation and maturation of viral mRNA. Inhibitors of this enzyme have shown efficacy at inhibiting vaccinia virus in vitro and in vivo (De Clercq et al. (1998) Nucleosides Nucleotides. 17:625-634.). Structurally, all active inhibitors reported to date are analogues of the nucleoside adenosine. Many are carbocyclic derivatives, exemplified by Neplanacin A and 3-Deazaneplanacin A. While these compounds have shown some efficacy in animal models, like many nucleoside analogues, they suffer from general toxicity and/or poor pharmacokinetic properties (Coulombe et al. (1995) Eur. J. Drug Metab Pharmacokinet. 20:197-202; Obara et al. (1996) J. Med. Chem. 39:3847-3852). It is unlikely that these compounds can be administered orally, and it is currently unclear whether they can act prophylactically against smallpox infections. Identification of non-nucleoside inhibitors of SAH hydrolase, and other chemically tractable variola virus genome targets that are orally bioavailable and possess desirable pharmacokinetic (PK) and absorption, distribution, metabolism, excretion (ADME) properties would be a significant improvement over the reported nucleoside analogues. In summary, currently available compounds that inhibit smallpox virus replication are generally non-specific and suffer from use limiting toxicities and/or questionable efficacies.
- In U.S. Pat. No. 6,433,016 (Aug. 13, 2002) and U.S. Application Publication 2002/0193443 A1 (published Dec. 19, 2002) a series of imidodisulfamide derivatives are described as being useful for orthopoxvirus infections.
- New therapies and preventatives are clearly needed for infections and diseases caused by orthopoxvirus infection.
- Several orthopoxviruses, including cowpox, monkeypox, camelpox, variola, and probably most other mammalian orthopoxviruses, can be grown readily in cell culture and produce robust cytopathic effect (CPE) in 3 to 5 days. Since this CPE is directly related to viral replication, compounds that inhibit virus replication in cell culture can be identified readily as conferring protection from virus-induced CPE (although it is theoretically possible to inhibit CPE without inhibiting virus replication). Moreover, compounds having identified activity against cowpox virus will also likely be active against human variola virus given the high degree of homology (>95%) between these two viruses and the fact that the replication proteins of orthopoxviruses are highly homologous. In general, the viruses diverge in regions of their genomes that encode immunomodulatory functions (host-specific). Additionally, many compounds have been identified in the literature that inhibit orthopoxvirus replication in cell culture and there are few, if any, examples where a compound is dramatically more potent against one species of orthopoxvirus and not the others
-
- wherein:
- R1 and R2 represent radicals independently selected from the group consisting of hydrogen and alkyl;
- R3 and R4 represent radicals independently selected from the group consisting of hydrogen and alkyl;
-
- R5 represents a radical selected from the group consisting of hydrogen and alkyl;
- R6 represents a radical selected from the group consisting of straight- or branched chain alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, cycloalkenyl, a substituted or unsubstituted aryl group, a substituted or unsubstituted heteroaryl group selected from the group consisting of furyl, thienyl, pyridyl, pyrrolyl, oxazolyl, thiazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, 1,2,3-oxadiazolyl, 1,2,3-triazolyl, and tetrazolyl; a substituted or unsubstituted arylalkyl group, and a substituted or unsubstituted heteroarylalkyl group, wherein the heteroaryl is selected from the group consisting pyridine and thiophene;
-
- said aryl group substituents and said arylalkyl group substituents being one or more radical(s) independently selected from the group consisting of a straight- or branched chain alkyl, alkoxy, alkoxyalkyl, alkoxyalkoxy, halogen, polyfluoroalkyl, polyfluoroalkoxy, carboxy, cyano, nitro, amido, amidoalkyl, carboxamide, alkylthio, alkylsulfinyl, alkylsulfonyl, sulfonamide, and mercapto;
- said heteroaryl group substituents and said heteroarylalkyl group substituents being one or more radical(s) independently selected from the group consisting of a straight- or branched chain alkyl, hydroxy, alkoxy, alkoxyalkyl, alkoxyalkoxy, halogen, polyfluoroalkyl, polyfluoroalkoxy, carboxy, cyano, amino, monoalkylamino, dialkylamino, aminoalkyl, nitro, amido, amidoalkyl, carboxamide, alkylthio, alkylsulfinyl, alkylsulfonyl, sulfonamide, and mercapto;
- or a pharmaceutically acceptable salt thereof.
- Also described herein are pharmaceutical compositions containing the antiviral compounds of Formula I and the corresponding methods of use for treating and preventing infections caused by orthopox viruses.
- Described herein are compounds of Formula I:
wherein R1, R2, R3, R4, R5, R6, and M are as defined above, with the proviso that said formula does include the compounds selected from the group consisting of N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl-4-pyridinecarboxamide; 4-bromo-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide; 3-bromo-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide; 3-chloro-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide; N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]-isoindol-2(1-yl)-4-pyridinecarboxamide; 4-bromo-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide; 4-methoxy-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide; 4-bromo-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethanocycloprop[f]isoindol-2(1H)-yl)-benzamide; 3-bromo-N-(1′,3′,3′a,4′,7′,7′a-hexahydro-1′,3′-dioxospiro[cyclopropane-1,8′-[4,7]methano[2H]isoindol]-2′-yl)-benzamide; N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-tricyclo[3.3.1.13,7]decane-1-carboxamide and 4-bromo-N-(1,3,3a,4,7,7a-hexahydro-1,3-dioxo-4,7-methano-2H-isoindol-2-yl)-benzamide. -
- R6 represents a radical selected from the group consisting of straight- or branched chain alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, cycloalkenyl, a substituted or unsubstituted aryl group, a substituted or unsubstituted heteroaryl group selected from the group consisting of furyl, thienyl, pyridyl, pyrrolyl, oxazolyl, thiazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, 1,2,3-oxadiazolyl, 1,2,3-triazolyl, and tetrazolyl; a substituted or unsubstituted arylalkyl group, and a substituted or unsubstituted heteroarylalkyl group, wherein the heteroaryl is selected from the group consisting pyridine and thiophene;
- said aryl group substituents and said arylalkyl group substituents being one or more radical(s) independently selected from the group consisting of a straight- or branched chain alkyl, alkoxy, alkoxyalkyl, alkoxyalkoxy, halogen, polyfluoroalkyl, polyfluoroalkoxy, carboxy, cyano, nitro, amido, amidoalkyl, carboxamide, alkylthio, alkylsulfinyl, alkylsulfonyl, sulfonamide, and mercapto;
- said heteroaryl group substituents and said heteroarylalkyl group substituents being one or more radical(s) independently selected from the group consisting of a straight- or branched chain alkyl, hydroxy, alkoxy, alkoxyalkyl, alkoxyalkoxy, halogen, polyfluoroalkyl, polyfluoroalkoxy, carboxy, cyano, amino, monoalkylamino, dialkylamino, aminoalkyl, nitro, amido, amidoalkyl, carboxamide, alkylthio, alkylsulfinyl, alkylsulfonyl, sulfonamide, and mercapto;
- or a pharmaceutically acceptable salt thereof.
-
- R6 represents a radical selected from the group consisting of straight- or branched chain alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, cycloalkenyl, a substituted or unsubstituted aryl group, a substituted or unsubstituted heteroaryl group selected from the group consisting of furyl, thienyl, pyridyl, pyrrolyl, oxazolyl, thiazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, 1,2,3-oxadiazolyl, 1,2,3-triazolyl, and tetrazolyl; a substituted or unsubstituted arylalkyl group, and a substituted or unsubstituted heteroarylalkyl group, wherein the heteroaryl is selected from the group consisting pyridine and thiophene;
- said aryl group substituents and said arylalkyl group substituents being one or more radical(s) independently selected from the group consisting of a straight- or branched chain alkyl, alkoxy, alkoxyalkyl, alkoxyalkoxy, halogen, polyfluoroalkyl, polyfluoroalkoxy, carboxy, cyano, nitro, amido, amidoalkyl, carboxamide, alkylthio, alkylsulfinyl, alkylsulfonyl, sulfonamide, and mercapto;
- said heteroaryl group substituents and said heteroarylalkyl group substituents being one or more radical(s) independently selected from the group consisting of a straight- or branched chain alkyl, hydroxy, alkoxy, alkoxyalkyl, alkoxyalkoxy, halogen, polyfluoroalkyl, polyfluoroalkoxy, carboxy, cyano, amino, monoalkylamino, dialkylamino, aminoalkyl, nitro, amido, amidoalkyl, carboxamide, alkylthio, alkylsulfinyl, alkylsulfonyl, sulfonamide, and mercapto;
- or a pharmaceutically acceptable salt thereof.
- Exemplary compounds include 4-trifluoromethyl-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide; 4-bromo-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethanocycloprop[f]isoindol-2(1H)-yl)-benzamide; 4-bromo-N-(octahydro-1,3-dioxo-2H-isoindol-2-yl)-benzamide; 4-fluoro-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide; 3-fluoro-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide; N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-1-2(1H)-yl)-4-pyridinecarboxamide; 4-bromo-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]-isoindol-2(1H)-yl)-benzamide; 4-chloro-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide; 4-trifluoromethyl-N-bicyclo[2.2.2]oct-5-ene-2,3-dicarboximido-benzamide; 4-trifluoromethyl-N-bicyclo[2.2.2]octane-2,3-dicarboximido-benzamide; and 2,4-dimethyl-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-thiazole-5-carboxamide.
- Also described herein compounds selected from the group consisting of 4-trifluoromethyl-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop-[f]isoindol-2(1H)-yl)-benzamide; 2-bromo-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide; N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-3-pyridinecarboxamide; N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-2-pyridinecarboxamide; 4-nitro-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide; 4-fluoro-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide; 3-fluoro-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide; 4-bromo-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethanocycloprop[f]isoindol-2(1H)-yl)-benzamide; 4-bromo-N-(1,3-(2H,3aH)-dioxo-4,8-ethenocyclohepta[c]pyrrolyl)-benzamide; 4-bromo-N-(octahydro-1,3-dioxo-2H-isoindol-2-yl)-benzamide; 4-bromo-N-bicyclo[2.2.2]oct-5-ene-2,3-dicarboximido-benzamide; 4-bromo-N-bicyclo[2.2.2]octane-2,3-dicarboximido-benzamide; 4-cyano-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]-isoindol-2(1H)-yl)-benzamide; 4-trifluoromethyl-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocy-cloprop[f]isoindol-2(1H)-yl)-benzamide; 4 trifluoromethyl-N-bicyclo[2.2.2]oct-5-ene-2,3-dicarboximido-benzamide; and 4-trifluoromethyl-N-bicyclo[2.2.2]octane-2,3-dicarboximido-benzamide.
- The compound may be selected from any of the compounds described, supra.
- Also described herein is a method for preventing and treating orthopoxvirus infections and for preventing and treating diseases associated with such infections in a living host (for example, a mammal including a human) having or susceptible to an orthopoxvirus infection, comprising the step of administering to the living host a therapeutically effective amount of a compound of the formula:
- wherein R1, R2, R3, R4, R5, R6, and M are as defined for compounds of Formula I above, or a pharmaceutically acceptable salt to a host susceptible to, or suffering from such infection.
- Such methods include the prevention and treatment of orthopoxvirus infections and diseases associated with such infections in a living host having or susceptible to an orthopoxvirus infection, comprising the step of administering a therapeutically effective amount of the compounds of the Formula Ia, above, or a pharmaceutically acceptable salt thereof. Also described is the prophylaxis or treatment of orthopoxvirus infections and diseases associated with such infections in a living host having or susceptible to an orthopoxvirus infection, comprising the step of administering a therapeutically effective amount of the compounds of the Formula Ib, above or a pharmaceutically acceptable salt, thereof.
- Also described herein are methods for the treatment or prevention of infections caused by an orthopox virus wherein the orthopox virus is selected from the group consisting of vaccinia virus, cowpox virus, smallpox (variola) virus, monkeypox virus and camelpox virus; in a living host (for example, a mammal including a human) comprising the step of administering a therapeutically effective amount of the compounds of the invention to a host susceptible to, or suffering from such infection.
-
- wherein R1, R2, R3, R4, R5, R6, and M are as defined for compounds of Formula I above, and a pharmaceutically acceptable carrier medium.
- The compounds described herein, their isomers and pharmaceutically acceptable salts, exhibit antiviral activity. The compounds described herein are particularly effective against orthopoxviruses, and are useful in the prophylaxis and/or treatment of infections and diseases associated with this virus in living hosts. Examples of orthopoxviruses that may be treated or prevented as described herein include, but are not limited to, aractuba virus, BeAn 58058 virus, buffalopox virus, camelpox virus (such as Camelpox virus 903, Camelpox virus CMG, Camelpox virus CMS, Camelpox virus CP1, Camelpox virus CP5, and Camelpox virus M-96), cantagalo orthopoxvirus, cowpox virus (such as Cowpox virus strain Hamburg-1985 and Cowpox virus strain Turkmenia-1974), Ectromelia virus (such as Belo Horizonte virus), elephantpox virus, monkeypox virus (such as Monkeypox virus strain Sierra Leone 70-0266 and Monkeypox virus strain Zaire 77-0666), rabbitpox virus (such as Rabbitpox strain Utrecht), raccoonpox virus, skunkpox virus, taterapox virus, vaccinia virus (including, but not limited to, the following strains: strain Ankara, strain Copenhagan, strain Dairen I, strain IHD-J, strain L-IPV, strain LC16M8, strain LC16MO, strain Lister, strain LIVP, strain Tian Tan, strain WR 65-16, strain WR, uand strain Wyeth), Variola virus (such as variola major virus and variola minor virus), and volepox virus.
- In vitro cell-based studies have been performed that demonstrate the usefulness of compounds described herein as antiviral agents. For example, antiviral activity of representative compounds was evaluated in assays that measure the ability of compounds to protect cells from virus-induced CPE. Cells that will support growth of the particular orthopox virus strain are seeded into 96-well tissue culture treated plates and then infected with an amount of the appropriate orthopox virus strain that results in complete CPE in ˜3 days. Various dilutions of inhibitory compound(s) are added and the plates are incubated at the appropriate temperature for optimal virus growth. At the end of the incubation period, cells are fixed with glutaraldehyde and stained with crystal violet. Cell protection is measured spectrophotometrically at OD570 nm. The interpolated compound dilution that results in 50% protection of the cell monolayer from virus-induced CPE is calculated and reported as the 50% effective concentration or EC50. Antiviral activity of representative compounds described herein occurred at drug concentrations that had no demonstrable effect on cell growth, indicating that the compounds were working specifically by an antiviral mechanism.
- The compounds described herein, collectively, include the compounds of Formula I, pharmaceutically acceptable salts thereof, their isomers, and mixtures thereof. The compounds are identified herein by their chemical structure and/or chemical name. Where a compound is referred to by both a chemical structure and a chemical name, and that chemical structure and chemical name conflict, the chemical structure is determinative of the compound's identity.
- The term “living host” as used herein refers to an organism that is living and capable of being infected with a virus, such as an orthopoxvirus; for example, a mammal, which includes a human.
- The term “alkyl” as used herein refers to straight or branched chain aliphatic hydrocarbon radicals of up to 10 carbon atoms, preferably up to 6 carbon atoms and more preferably 1 to 4 carbon atoms. Similarly, the term “alkyl”, or any variation thereof, used in combination form to name substituents, such as alkoxy (—O-alkyl), allylthio (—S-alkyl), monoalkylamino (—NH-alkyl), dialkylamino (—N-(alkyl)alkyl), alkylsulfonyl (—S(O)2-alkyl), carboxyalkyl (-alkyl-COOH), or the like, also refers to aliphatic hydrocarbon radicals of one to six carbon atoms, and preferably of one to four carbon atoms. Also “alk” in structural formula denotes an alkyl group, unless divalency is indicated in which case the “alk” denotes the corresponding alkylene group(s). Additionally, the term “lower alkyl” denotes an alkyl group having one to four carbon atoms.
- The term “alkenyl” as used herein refers to straight or branched chain aliphatic hydrocarbon radicals of 2 to 7 carbon atoms containing one double bond. Such alkenyl moieties may exist in the E or Z configurations; the compounds of this invention include both configurations. The term “alkynyl” as used herein refers to straight or branched chain aliphatic hydrocarbon radicals containing 2 to 7 carbon atoms having at least one triple bond.
-
- As used herein, the term “aryl”, when used as such, refers to an aromatic carbocyclic group, having 6 to 10 carbon atoms including without limitation phenyl and napthyl.
- The term “heteroaryl,” as used herein, refers to a 5- or 6-membered aromatic cyclic group having at least one carbon atom and one or more oxygen, nitrogen or sulfur atoms in the ring, as for example furyl, thienyl, pyridyl, pyrrolyl, oxazolyl, thiazolyl, imidazolyl, pyrazolyl, isoxazolyl, isothiazolyl, 1,2,3-oxadiazolyl, 1,2,3-triazolyl, tetrazolyl, and the like, including all position isomers. Preferred heteroaryl groups include pyridine, thiazole and thiophene.
- As used herein, the term “cycloalkyl” refers to a saturated hydrocarbon ring. Cycloalkyls can be monocyclic or can be fused, spiro or bridged bicyclic or tricyclic ring systems. Monocyclic cycloalkyl rings contain from 3 to 10 carbon atoms, preferably from 3 to 7 carbon atoms, as for example cyclopropyl, cyclobutyl, cyclopentyl, and cyclohexyl. Bicyclic and tricyclic cycloalkyl rings contain from 7 to 28 carbon atoms, preferably from 7 to 19 carbon atoms, in the ring system; and include, for example, adamantyl, bicyclo[2.2.1]heptyl, bicyclo[2.2.2]cyclooctanyl, tricyclo[3.2.2.02,4]nonyl and norbornyl, and bicyclo[3.2.2]nonyl. As used herein, the term “cycloalkenyl” refers to an unsaturated hydrocarbon ring. Cycloalkenyl rings are non-aromatic and contain at least one (preferably only one) carbon-carbon double bond. Cycloalkenyl rings are monocyclic, or are fused, spiro or bridged bicyclic or tricyclic ring systems. Monocyclic cycloalkenyl rings contain from 5 to 10 carbon atoms, preferably from 5 to 7 carbon atoms, and include, for example, cyclopropenyl, cyclobutenyl, cyclopentenyl, and cyclohexenyl. Bicyclic and tricyclic cycloalkenyl rings contain from 7 to 28 carbon atoms in the ring, preferably from 7 to 19 carbon atoms, in the ring system; and include, for example, bicyclo[2.2.1]hept-2-ene, bicyclo[2.2.2]cyclooct-2-enyl, tricyclo[3.2.2.02,4]non-6-enyl, and bicyclo[3.2.2]non-6-enyl.
- The term “amido,” as used herein, refers to a radical or substituent of the formula—NR″C(═O)R′″, wherein R″ and R′″ represent hydrogen or alkyl.
- The term “carboxamide,” as used herein, refers to a radical or substituent of the formula —C(═O)—NR″R′″, wherein R″ and R′″ are as previously defined.
- The term “sulfonamide,” as used herein, refers to a radical or substituent of the formula —SO2NR″R′″ or —NR″SO2R′″, wherein R″ and R′″ are as previously defined.
- The term “halogen,” as used herein, refers to a radical or substituent selected from the group consisting of chloro, bromo, iodo, and fluoro.
- The term “HPLC,” as used herein, refers to high-performance liquid chromatography.
- “Substituted” is intended to indicate that one or more hydrogens on the atom indicated in the expression using “substituted” is replaced with a selection from the indicated group(s), provided that the indicated atom's normal valency is not exceeded, and that the substitution results in a stable compound: When a substituent is an oxo (═O) group, then 2 hydrogens on the atom are replaced.
- The compounds described herein and their pharmaceutically acceptable salts are useful in treating and preventing viral infections and diseases in living hosts when used in combination with other active agents, including but not limited to interferons, ribavirin, immunoglobulins, immunomodulators, anti-inflammatory agents, antibiotics, antivirals, anti-infectious agents, and the like.
- Compounds described herein are also useful in preventing or resolving orthopox viral infections in cell, tissue or organ cultures and other in vitro applications. For example, inclusion of compounds of the invention as a supplement in cell or tissue culture growth media and cell or tissue culture components will prevent viral infections or contaminations of cultures not previously infected with viruses. Compounds described above may also be used to eliminate or attenuate viral replication in cultures or other biological materials infected or contaminated with viruses (for example, blood), after a suitable treatment period, under any number of treatment conditions as determined by the skilled artisan.
- The compounds described herein can form useful salts with inorganic and organic acids such as hydrochloric, sulfuric, acetic, lactic, or the like and with inorganic or organic bases such as sodium or potassium hydroxide, piperidine, ammonium hydroxide, or the like. The pharmaceutically acceptable salts of the compounds of Formula I are prepared following procedures that are familiar to those skilled in the art.
- The phrase “pharmaceutically acceptable” is employed herein to refer to those compounds, materials, compositions, and/or dosage forms which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of human beings and animals without excessive toxicity, irritation, allergic response, or other problem or complication commensurate with a reasonable benefit/risk ratio.
- To the extent that certain compounds described herein may have at least one chiral center, the compounds may thus exist as enantiomers. In addition, the compounds described herein may also possess two or more chiral centers and thus may also exist as diastereomers or as exo or endo isomers. Where the processes for the preparation of the present compounds give rise to a mixture of stereoisomers, these isomers may be separated by conventional techniques such as preparative chromatography. Accordingly, the compounds may be prepared as a racemic mixture or, by either enantiospecific synthesis or resolution, as individual enantiomers. The compounds may, for example, be resolved from a racemic mixture into their component racemates by standard techniques, such as the formation of diastereomeric pairs by salt formation with an optically active acid, such as (−)-di-p-toluoyl-d-tartaric acid and/or (+)-di-p-toluoyl-1-tartaric acid followed by fractional crystallization and regeneration of the free base. The racemic mixture may also be resolved by formation of diastereomeric esters or amides, followed by chromatographic separation and removal of the chiral auxiliary. Alternatively, the compounds may be resolved using a chiral HPLC column. It is to be understood that all such isomers and mixtures thereof are encompassed within the scope described herein.
- The compounds described herein are useful for treating orthopoxvirus infection in living hosts, for example, mammals including humans. When administered to a living host the compounds can be used alone, or as a pharmaceutical composition.
- Pharmaceutical compositions comprising the compounds described herein, either alone or in combination with each other, offer a treatment against orthopoxvirus infection. The antiviral pharmaceutical compositions described herein comprise one or more of the compound(s) of Formula I above, as the active ingredient in combination with a pharmaceutically acceptable carrier medium or auxiliary agent.
- The composition may be prepared in various forms for administration, including tablets, caplets, pills or dragees, or can be filled in suitable containers, such as capsules, or, in the case of suspensions, filled into bottles. As used herein, “pharmaceutically acceptable carrier medium” includes any and all solvents, diluents, or other liquid vehicle, dispersion or suspension aids, surface active agents, isotonic agents, thickening or emulsifying agents, preservatives, solid binders, lubricants and the like, as suited to the particular dosage form desired. Remington's Pharmaceutical Sciences, Twentieth Edition, A. R. Gennaro (William and Wilkins, Baltimore, Md., 2000) discloses various carriers used in formulating pharmaceutical compositions and known techniques for the preparation thereof. Except insofar as any conventional carrier medium is incompatible with the antiviral compounds of the invention, such as by producing any undesirable biological effect or otherwise interacting in a deleterious manner with any other component(s) of the pharmaceutical composition, its use is contemplated to be within the scope of the compositions described herein.
- A pharmaceutical composition may include one or more water-insoluble diluents, water-soluble diluents, disintegrants, binders, wetting agents, solubilizers, glidants, lubricants, granulating solvents. An example of a suitable water-insoluble diluent is microcrystalline cellulose. An example of a suitable water-soluble diluent is lactose monohydrate. An example of a suitable typical disintegrant is croscarmellose sodium. An example of a suitable binder is hydroxypropylmethyl cellulose. An example of a suitable glidant is colloidal silicone dioxide. An example of a suitable lubricant is magnesium stearate. An example of a suitable granulating solvent is water.
- In the pharmaceutical compositions described herein, the active agent may be present in an amount of at least 0.5% and generally not more than 90% by weight, based on the total weight of the composition, including carrier medium and/or auxiliary agent(s), if any. Typically, the proportion of active agent varies between 5 to 50% by weight of the composition.
- Pharmaceutical organic or inorganic solid or liquid carrier media suitable for enteral or parenteral administration can be used to make up the composition. Gelatine, lactose, starch, magnesium stearate, talc, vegetable and animal fats and oils, gum, polyalkylene glycol, or other known medicament components may all be suitable as carrier media or excipients.
- The compounds described herein may be administered using any amount and any route of administration effective for attenuating infectivity of the virus. Thus, the expression “amount effective to attenuate infectivity of virus,” as used herein, refers to a nontoxic but sufficient amount of the antiviral agent to provide the desired prophylaxis and/or treatment of viral infection. The exact amount required will vary from subject to subject, depending on the species, age, and general condition of the subject, the severity of the infection, the particular antiviral agent, its mode of administration, and the like.
- The compounds described herein may be administered within 24 hours of symptom onset, although therapeutic effects may be produced with administration within 48 hours of symptom onset, or even within 72 hours of symptom onset. Symptoms of initial orthopoxvirus infections depend on the exact virus contracted. For example, the initial symptoms of a smallpox infection include fever, malaise, head and body aches, and sometimes vomiting.
- The antiviral compounds may be formulated in dosage unit form for ease of administration and uniformity of dosage. “Dosage unit form,” as used herein, refers to a physically discrete unit of antiviral agent appropriate for the patient to be treated. Each dosage should contain the quantity of active material calculated to produce the desired therapeutic effect either as such, or in association with the selected pharmaceutical carrier medium and/or the supplemental active agent(s), if any. Typically, the antiviral compounds of the invention will be administered in dosage units containing from about 100 mg to about 2,000 mg of the antiviral agent by weight of the composition, although dosage units containing from about 10 mg up to about 10,000 mg may also be employed.
- The compounds may be administered orally, rectally, parenterally, such as by intramuscular injection, subcutaneous injection, intravenous infusion or the like, intracisternally, intravaginally, intraperitoneally, locally, such as by powders, ointments, or drops, or the like, or by inhalation, such as by aerosol or the like, taking into account the nature and severity of the infection being treated. Depending on the route of administration, the compounds of the invention may be administered at dosage levels of about 0.125 to about 250 mg/kg of subject body weight per dose, one or more times a day, to obtain the desired therapeutic effect.
- The compounds of the invention will typically be administered from 1 to 4 times a day so as to deliver the above-mentioned daily dosage. However, the exact regimen for administration of the compounds and compositions described herein will necessarily be dependent on the needs of the individual host or patient being treated, the type of treatment administered and the judgment of the attending medical specialist.
- For prophylaxis, compounds of the invention may be administered within 48 hours after possible exposure, although effective prophylaxis can be produced by administration within 7 days post exposure, up to as long as 14 days post exposure. The dosages may be essentially the same, whether for treatment or prophylaxis of virus infection.
- During any of the processes for preparation of the compounds described herein, it may be necessary and/or desirable to protect sensitive or reactive groups on any of the molecules concerned. This may be achieved by means of conventional protecting groups, such as those described in Protective Groups in Organic Chemistry, ed. J. F. W. McOmie, Plenum Press, 1973; and T. W. Greene & P. G. M. Wuts, Protective Groups in Organic Synthesis, John Wiley & Sons, 1999. The protecting groups may be removed at a convenient subsequent stage using methods known from the art.
- The following examples are provided to describe the invention in further detail. These examples illustrate suitable methods of synthesis of representative compounds of this invention. However, the methods of synthesis are intended to illustrate and not to limit the invention to those exemplified below. The starting materials for preparing the compounds of the invention are either commercially available or can be conveniently prepared according to one of the examples set forth below or otherwise using known chemistry procedures.
-
- A mixture of cycloheptatriene (5 g, 54.26 mmol) and maleic anhydride (6.13 g, 62.40 mmol) in xylenes (35 mL) was heated at reflux under argon overnight. The reaction was cooled to room temperature and a tan precipitate was collected by filtration and dried to give 2.94 grams (28%) of the desired product.
- b. Preparation of ST-246. A mixture of compound I (a) (150 mg, 0.788 mmol) and 4-trifloromethylbenzhydrazide (169 mg, 0.827 mmol) in ethanol (10 mL) was heated under argon overnight. The solvent was removed by rotary evaporation. Purification by column chromatography on silica gel using 1/1 hexane/ethyl acetate provided 152 mg (51%) of the product as a white solid.
- The compounds of Examples 2-14 were synthesized following the above mentioned general procedure for Example 1 using compound I (a) and reacting it with the following hydrazides: isonicotinic hydrazide, 4-bromobenzoic hydrazide, 3-bromobenzoic hydrazide, 3-chlorobenzoic hydrazide, 2-bromobenzoic hydrazide, 2-chlorobenzoic hydrazide, 4-chlorobenzoic hydrazide, nicotinic hydrazide, 2-picolinyl hydrazide, 4-methoxybenzoic hydrazide, 4-nitrobenzoic hydrazide, 4-fluorobenzoic hydrazide, and 3-fluorobenzoic hydrazide.
-
- To a solution of compound 1(a) (1 g, 5.26 mmol) in ethanol (20 mL) was added 10% palladium on activated carbon (100 mg, 10 wt %). The mixture was shaken on a Parr hydrogenator under an atmosphere of hydrogen at 50 psi for 3 hours. The mixture was filtered through a micron filter to remove the palladium, and the filtrate was concentrated to give 384 mg (38%) of the product as a white solid.
- b. Preparation of 4-bromo-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethanocycloprop[f]-isoindol-2(1H)-yl)-benzamide. A mixture of compound 15(a) (350 mg, 1.82 mmol) and 4-bromobenzoic hydrazide (411 mg, 1.91 mmol) in ethanol (10 mL) was heated under argon for 48 hours. The solvent was removed by rotary evaporation. Purification by column chromatography on silica gel using 1/1 hexane/ethyl acetate as eluent provided 444 mg (63%) of the product as a white solid.
-
- A mixture of 1,3-cycloheptadiene (0.87 mL, 10.62 mmol) and maleic anhydride (1.2 g, 12.24 mmol) in xylenes (7 mL) was heated at reflux under argon overnight. The reaction was cooled to room temperature, and a tan precipitate was collected by filtration and dried to give 1.59 grams (78%) of the desired product.
- b. Preparation of 4-bromo-N-(1,3-(2H,3aH)-dioxo-4,8-ethenocyclohepta[c]prrolyl)-benzamide. A mixture of compound 16(a) (500 mg, 2.6 mmol) and 4-bromobenzoic hydrazide (587 mg, 2.73 mmol) in ethanol (5 mL) was heated under argon overnight. The solvent was removed by rotary evaporation. Purification by column chromatography on silica gel using 1/1 hexane/ethyl acetate provided 683 mg (67%) of the product as a white solid.
- A mixture of cis-cyclohexanedicarboxylic anhydride (150 mg, 0.97 mmol) and 4-bromobenzoic hydrazide (220 mg, 1.02 mmol) in ethanol (10 mL) was heated under argon overnight. The solvent was removed via rotary evaporation. Purification by column chromatography on silica gel using 1/1 hexane/ethyl acetate as eluent provided 179 mg (52%) of the desired product as a white solid.
-
- A mixture of 1,3-cyclohexadiene (2.4 mL, 24.96 mmol) and maleic anhydride (2.81 g, 28.66 mmol) in xylenes (15 mL) was heated at reflux overnight. The solution was cooled to room temperature and the precipitate was collected by suction filtration. The solid was washed with xylenes and dried to give 3.08 g (69%) of the product as a tan solid.
- b. Preparation of compound 4-bromo-N-bicyclo[2.2.2]oct-5-ene-2,3-dicarboximido-benzamide. A mixture of compound 18(a) (150 mg, 0.84 mmol) and 4-bromobenzoic hydrazide (190 mg, 0.88 mmol) in ethanol (10 mL) was heated under argon overnight. The solvent was removed by rotary evaporation. Purification by column chromatography on silica gel using 1/1 hexane/ethyl acetate gave 210 mg (67%) of the product as a white solid.
- (See Tables 1 and 2 below for listed compound names and structures)
- A mixture of compound 1(a)(150 mg, 0.788 mmol) and 2,4-dimethylthiazole-5-carboxylic acid hydrazide (141 mg, 0.827 mmol) in ethanol (10 mL) was heated at reflux under argon overnight. The solution was then cooled to room temperature, and the white precipitate was collected by filtration. The solid was washed with ethanol, and air-dried affording 183 mg (68%) of the product as a white solid.
- By appropriate selection of suitable starting materials, other compounds of the invention may be prepared according to the procedures described in the foregoing examples. Representative examples of further di, tri, and tetracyclic acylhydrazide derivatives and analogues are set forth in Tables 1 and 2 below.
TABLE 1 Example **Mass Number R6 *NMR Spec Name 1 1HNMR DMSO-d6: δ11.35 (d, 1H): 11.09 (d, 1H); 8.08 (d, 2H); 7.92 (d, 2H); 5.799 (s, 2H); 3.29 (brs, 4H), 1.17 (m, 2H); 0.26 (m, 1H; 0.078 (s, 1H) 375 (M − H)− 4-Trifluoromethyl-N- (3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- ethenocycloprop[f] # isoindol- 2(1H)-yl)-benzamide 2 1HNMR in DMSO-d6: δ11.41 (brs); 11.15 (brs); 8.77 (d of d, 2H); 7.75 (d, 2H); 5.77 (brs, 2H), 3.27 (brs, 4H), 1.15 (brs, 2H); 0.25 (m, 1H; 0.03 (brs, 1H) 308 (M − H)− N-(3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- ethenocycloprop[f] # isoindol- 2(1H)-yl)-4- pyridinecarboxamide 3 *** 385 (M − H)− 4-Bromo-N- (3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)-benzamide 4 1HNMR in DMSO-d6: δ11.13 (brd, 1H); 10.89 (brd, 1H); 7.99 (s, 1H); 7.92-7.76 (m, 2H); 7.43 (t, 1H); 5.72 (s, 2H), 3.22- 3.08 (m, 4H); 1.19 (brs, 2H; 0.21 (m, 1H); 0.17 (brs, 1H) 385 (M − H)− 3-Bromo-N- (3,3a,4,4a,5,5a,6,6a,- # octahydro-1,3-dioxo-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)-benzamide 5 1HNMR in DMSO-d6: δ11.21 (brd, 1H); 10.98 (brd, 1H); 7.92 (s, 1H); 7.85 (d, 1H); 7.71 (d, 1H); 7.58 (t, 1H), 5.79 (brs, 2H); 3.29-3.15 (m, 4H); 1.19- 1.15 (m, 2H); 0.26 (m, 1H); 0.10 (brs, 1H) 341 (M − H)− 3-Chloro-N- (3,3a,4,4a, # 5,5a,6,6a,- octahydro-1,3-dioxo-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)-benzamide 6 1HNMR in CDCl3: δ7.74 (s, 1H); 7.69 (d, 1H); 7.63 (d, 1H); 7.41-7.31 (m, 2H); 5.84 (m, 2H); 3.48 (m, 2H), 3.14 (s, 2H); 1.19 (m, 2H); 0.38- 0.20 (m, 2H) 385 (M − H)− 2-Bromo-N- (3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- # ethenocycloprop[f]isoindol- 2(1H)-yl)-benzamide 7 1HNMR in CDCl3: δ7.96 (s, 1H); 7.83 (d, 1H); 7.45 (m, 2H); 7.36 (m, 1H); 5.86 (d, 2H); 3.47 (brs, 2H), 3.15 (s, 2H); 1.15 (brs, 2H); 0.39-0.20 (m, 2H) 341 (M − H)− 2-Chloro-N- (3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- # ethenocycloprop[f]isoindol- 2(1H)-yl)-benzamide 8 1HNMR in DMSO-d6: δ11.16 (brd, 1H); 10.91 (brd, 1H); 7.90 (d, 2H); 7.61 (d, 2H); 5.79 (s, 2H); 3.28 (m, 4H), 1.17 (s, 2H); 0.26 (m, 1H); 0.07 (s, 2H) 341 (M − )H− 4-Chloro-N- (3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- ethenocycloprop[f] # isoindol- 2(1H)-yl)-benzamide 9 1HNMR in DMSO-d6: δ11.33 (brd, 1H); 9.04 (s, 1H); 8.8 (m, 1H); 8.23 (d, 1H); 7.56 (m, 1H); 5.80 (s, 2H); 3.29 (m, 4H); 1.17 (m, 2H); 0.27 (m, 1H); 0.07 (s, 1H) 308 (M − )H− N-(3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- ethenocycloprop[f] # isoindol- 2(1H)-yl)-3- pyridinecarboxamide 10 1HNMR in DMSO-d6: δ11.11 (s, 1H); 8.70 (d, 1H); 8.07-8.02 (M, 2H); 7.7- 7.66 (m, 1H); 5.75 (m, 2H); 3.295 (s, 4H), 1.16 (m, 2H); 0.27 (m, 1H); 0.10 (s, 1H) 308 (M − H)− N-(3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- ethenocycloprop[f] # isoindol- 2(1H)-yl)-2- pyridinecarboxamide 11 1HNMR in DMSO-d6: δ10.87 (brd, 1H); 7.87 (d, 2H); 7.05 (d, 2H); 5.78 (br, 2H); 3.84 (s, 3H); 3.30 (s, 4H); 1.16 (m, 2H); 0.25 (m, 1H); 0.07 (brs, 1H) 339 (M + H)+ 4-Methoxy-N- (3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- # ethenocycloprop[f]isoindol- 2(1H)-yl)-benzamide 12 1HNMR in DMSO-d6: δ11.537- 11.469 (brd, 1H); 8.38 (d, 2H); 5.80 (s, 2H); 3.3 (br, 4H); 1.18 (s, 2H); 0.27 (m, 1H); 0.08 (s, 1H) 352 (M −H)− 4-Nitro-N- (3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- ethenocycloprop[f]isoindol- # 2(1H)-yl)-benzamide 13 1HNMR in DMSO-d6: δ11.04 (br, 1H); 7.96 (s, 2H); 7.367 (t, 2H); 5.791 (s, 2H); 3.258 (4H & H2O), 1.18 (d, 2H); 0.28 (m, 1H); 0.09 (s, 1H) 327.0 (M + H)+ 4-Fluoro-N- (3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- # ethenocycloprop[f]isoindol- 2(1H)-yl)-benzamide 14 1HNMR in DMSO-d6: δ11.176 (br, 1H); 7.768- 7.459 (m, 4H); 5.797 (s, 2H); 3.293 (H2O & 4H), 1.174 (s, 2H); 0.23 (m, 1H); 0.05 (s, 1H) 327.0 (M + H)+ 3-Fluoro-N- (3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- ethenocycloprop[f] # isoindol- 2(1H)-yl)-benzamide 15 *** 388.9 (M − H)− 4-Bromo-N-(octahydro-1,3- dioxo-4,6- ethanocycloprop[f]isoindol- 2(1H)-yl)-benzamide 16 1HNMR in DMSO-d6: δ11.14 (brd, 1H); 7.85 (brd, 2H); 7.76 (d, 2H); 6.10 (brs, 2H) 3.43 (brd, 2H), 2.86 (brs, 2H); 1.98- 1.54 (m, 6H) 387 (M − H)− 4-Bromo-N-(1,3-(2H, 3aH)- dioxo-4,8- ethanocycloprop[c]pyrrolyl)- benzamide 17 1HNMR in DMSO-d6: δ11.16 (s, 1H); 7.86 (d, 2H); 5.797 (s, 2H); 3.293 (H2O & 4H), 1.174 (s, 2H); 0.23 (m, 1H); 0.05 (s, 1H) 350.9 (M + H)+ 4-Bromo-N-(octahydro-1,3- dioxo-2H-isoindol-2-yl)- benzamide 18 1HNMR in DMSO-d6: δ11.05 (brd, 1H); 7.83 (d, 2H); 7.76 (d, 2H); 6.21 (s, 2H), 3.15 (s, 2H); 3.04 (s, 2H); 1.66 (d, 2H); 1.28 (d, 2H) 373 (M − H)− 4-Bromo-N- bicyclo[2.2.2]oct-5-ene-2,3- dicarboximido-benzamide 19 1HNMR in DMSO-d6: δ11.15 (s, 1H); 7.87 (d, 2H); 7.78 (d, 2H); 3.07 (m, 2H), 2.04 (s, 2H); 1.75-1.64 (m, 2H); 1.45- 1.38 (m, 3H) 373 (M − H)− 4-Bromo-N- bicyclo[2.2.2]octane-2,3- dicarboximido-benzamide 20 1HNMR in DMSO-d6: δ11.36 (br, 1H); 8.03 (s, 4H); 5.79 (s, 2H); 3.30 (4H +H2O); 2.50 (s, 2H); 1.20 (s, 2H) 332.1 (M − H)− 4-Cyano-N- (3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)-benzamide 21 1HNMR in DMSO-d6: δ11.286 (br, 1H); 8.13 (d, 2H); 8.10 (d, 2H); 3.30 (4H +H2O);1.49-1.12 (m, 4H); 0.83 (s, 1H); 0.57 (s, 1H) 377.0 (M − H)− 4-Trifluoromethyl-N- (3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- ethanocycloprop[f]isoindol- # 2(1H)-yl)-benzamide 22 *** *** 4-Methyl-N- (3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- ethanocycloprop[f]isoindol- 2(1H)-yl)-benzamide 23 *** *** 3-Bromo-N- (1′,2,2′a,4′,7,7′a-hexahydro- 1′,3′,dioxospiro [cyclopropane-1,8′-[4,7]methano[2H]isoindol]-2′-yl)- benzamide 24 *** *** N-(3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)-tricyclo[3.3.1.13.7]decane-1-carboxamide 25 *** *** N-(3,3a,4,4a,5,5a,6,6a,- octahydro-1,3-dioxo-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)- benzeneacetamide 26 *** *** 4-Bromo-N-(1,3,3a,4,7,7a- hexahydro-1,3,-dioxo-4,7- methano-2H-isoindol-2-yl)- benzamide 27 *** *** 2,4-Dichloro-N- (1,3,3a,4,7,7a-hexahydro- 1,3,-dioxo-4,7-methano-2H- isoindol-2-yl)- benzamide 28 1HNMR in DMSO-d6: δ11.37 (br, 1H); 8.10 (d, 2H); 7.94 (d, 2H); 6.22 (s, 2H); 3.17 (s, 2H); 3.05 (s, 2H); 1.66 (m, 2H); 1.29 (m, 2H) 365.0 (M + H)+ 4-Trifluoromethyl-N-bicylo [2.2.2]oct-5-ene-2,3- dicarboximido-benzamide 29 1HNMR in DMSO-d6: δ11.33 (s, 1H); 8.14 (d, 2H); 8.11 (d, 2H); 3.29 (s, 4H); 2.05 (s, 2H); 1.76- 1.65 (m, 4H); 1.42 (s, 2H) 367.0 (M + H)+ 4-Trifluoromethyl-N-bicyclo [2.2.2]octane-2,3- dicarboximido-benzamide
*All 1H NMR and 13C NMR spectra were acquired on a Varian Mercury VX300 Spectrometer and referenced to tetramethylsilane (TMS) unless indicated otherwise.Chemical shifts and coupling constants are reported in parts per million (ppm) and Hertz(Hz), respectively, Multiplicities indicated are: s = singlet, d = doublet, t = triplet, q = quartet,m = multiplet, dd = doublet of doublets, and br indicates a broad signal.
**Mass Spectroscopy data is expressed as a mass to charge ratio (m/z) for either (M + 1) or (M-1) molecular ion.
***indicates that data were not collected.
- The following table contains further examples of compounds of the invention, which may be prepared as exemplified above and/or may be synthesized according to the previous procedures or otherwise using conventional chemistry knowledge.
TABLE 2 Example Number Structure Name 30 4-Trifluoromethyl-N- (3,3a,4,4a,5,5a,6,6a- octahydro-1,3-dioxo-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)-N- methylbenzamide 31 4-Trifluoromethyl-N- (3,3a,4,4a,5,5a,6,6a- octahydro-1,3-dioxo-4,6- ethenocycloprop[f)isoindol- 2(1H)-yl)-N-ethylbenzamide 32 4-Trifluoromethyl-N- (3,3a,4,4a,5,5a,6,6a- octahydro-1,3-dioxo-7,8- dimethyl-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)-benzamide 33 4-Trifluoromethyl-N-(3a,4,7, 7a-tetrahydro-4,7-etheno- 1H-isoindol-2(1H)-yl)- benzamide 34 N-(3,3a,4,4a,5,5a,6,6a- octahydro-1,3-dioxo-7,8- dimethyl-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)-acetamide 35 N-(3,3a,4,4a,5,5a,6,6a- octahydro-1,3-dioxo-7,8- dimethyl-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)-but-3-enamide 36 N-(3,3a,4,4a,5,5a,6,6a- octahydro-1,3-dioxo-7,8- dimethyl-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)- cyclohexanecarboxamide 37 4-Trifluoromethyl-N- (3,3a,4,4a,5,5a,6,6a- octahydro-1,3-dioxo-7,8- dimethyl-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)-benzylacetamide 38 4-Pyridyl-N- (3,3a,4,4a,5,5a,6,6a- octahydro-1,3-dioxo-7,8- dimethyl-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)-acetamide 39 3-Thienyl-N- (3,3a,4,4a,5,5a,6,6a- octahydro-1,3-dioxo-7,8- dimethyl-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)-N- methylbenzamide 40 N-[(3aR,4R,4aR,5aS,6S,6aS)- 3,3a,4,4a,5,5a,6,6a- octahydro-1,3-dioxo-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl]-4-(trifluoromethyl)- benzamide 41 2,4-Dimethyl-N- (3,3a,4,4a,5,5a,6,6a- octahydro-1,3-dioxo-4,6- ethenocycloprop[f]isoindol- 2(1H)-yl)-thiazole-5- carboxamide
Inhibition of Orthopox Viral Replication - The ability of the compounds described herein to inhibit Vaccinia virus was established by the following experimental procedure:
- (a) Preparation of Virus Stock:
- Virus stocks of Vaccinia virus (NYCBH) were prepared in Vero cells infected at low multiplicity (0.01 plaque forming units (PFU)/cell) and harvested when cytopathic effects were complete (4+CPE). The samples were frozen and thawed and then sonicated to release cell-associated virus. The cell debris was removed by low-speed centrifugation, and the resulting virus suspension was stored in 1 mL aliquots at −80° C. The PFU/mL of the virus suspension was quantified by standard plaque assay on Vero and BSC-40 cells.
- (b) Vaccinia CPE: Assay:
- To determine the amount of vaccinia virus stock required to produce complete CPE in 3 days, Vero cell monolayers were seeded on to 96-well plates and infected with 2-fold serial dilutions of the vaccinia virus stock. At 3 days post-infection, the cultures were fixed with 5% glutaraldehyde and stained with 0.1% crystal violet. Virus-induced CPE was quantified spectrophotometrically at OD570. From this analysis, a 1:800 dilution of vaccinia virus stock was chosen for use in the HTS assay. This amount of vaccinia virus represents a multiplicity of infection of approximately 0.1 PFU/cell. To establish the signal-to-noise ratio (S/N) of the 96-well assay and evaluate the well-to-well and assay-to-assay variability, six independent experiments were performed. Vero cell monolayers were infected with 1:800 dilution of vaccinia virus stock. Each plate contained the following controls: quadruplicate virus-infected wells, quadruplicate uninfected cell wells and a dose response curve in duplicate for cidofovir (CDV) added at 300, 100, 30 and 10 DAM, or phosphonoacetic acid (PAA) added at 2100, 714, 210, and 71.μM as reference standards. At day 3 post-infection, the plates were processed as described above.
- The results of these experiments indicated that the 96-well assay format is robust and reproducible. The S/N ratio (ratio of signal of cell control wells (signal) to virus control wells (noise)) was 9.2±1.8. The well-to-well and assay-to-assay variability was less than 20%. Using this assay, the EC50 values for CDV and PAA were determined to be 84±15 μM and 985±85 μM, respectively. These values were within the range of published values for these compounds. Based on this analysis, the 1:800 dilution of vaccinia virus (boxed) was chosen for use in the assay.
- (c) Compound Testing:
- Representative compounds of the invention were tested in the vaccinia virus CPE assay. Compounds were dissolved in DMSO and diluted in medium such that the final concentration in each well was 5 μM compound and 0.5% DMSO. The compounds were added robotically to the culture medium using the Biomek.RTM. FX robot system. Following compound addition, the cultures were infected with vaccinia virus. After 3 days, plates were processed and CPE quantified as described.
- Representative compounds of the invention inhibited vaccinia virus-induced CPE by great than 50% at the test concentration (5 μM). Selected compounds were further evaluated for potency (EC50) in the CPE assay and cytotoxicity (CC50) in an MTT assay. The MTT assay measures mitochondrial dehydrogenase activity in dividing cells. This method detects the in situ reduction of (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl-)-2H-tetrazolium) using an electron coupling reagent (phenazine methosulfate) to produce an insoluble formazan. The absorbance of the formazan at 490 nm can be measured directly from 96-well assay plates following solubilization of the formazan in 50% ethanol. The quantity of formazan product is directly proportional to the number of living cells in culture.
- EC50 values are determined by comparing compound-treated and compound-untreated cells using a computer program. (The EC50 value measures compound concentration that inhibits viral replication by 50%). The EC50 values of representative compounds of the invention in the CPE assay are listed in Table 3, below. These compounds were active at non-toxic concentrations.
TABLE 3 Vaccinia EC50 Cowpox EC50 A = <0.5 μM, A = <0.5 μM, B = 0.5 to <1.0 μM, B = 0.5 to <1.0 μM, Example C = 1.0 to <5 μM, C = 1.0 to <5 μM, Number D = ≧5 μM D = ≧5 μM 1 A A 2 A C 3 A B 4 A C 5 B B 6 B D 7 A *** 8 A B 9 A D 10 D D 11 C D 12 A A 13 A B 14 A C 15 A A 16 A A 17 A C 18 A A 19 A A 20 A A 21 A A 22 A C 23 A *** 24 A B 25 B D 26 A *** 27 B *** 28 A A 29 A A 41 A C
*** Indicates that data were not collected.
- Several additional CPE inhibition assays, similar to above, were utilized to identify a spectrum of activity of compounds of the invention within the orthopox genus. For example, the corresponding EC50 values of representative compounds in wild type cowpox virus (obtained from USAMRIID, Fort Detrick, Frederick, Md.) are listed in Table 3, above.
- Table 4 lists EC50 values of select compounds of the invention measuring anti-orthopox virus activities in these CPE inhibition assays for cidofovir-resistant cowpox virus (Brighton Red strain, (available from USAMRIID Fort Detrick, Frederick, Md.), camelpox, and monkeypox virus (Zaire(V79-1-005-scab)).
TABLE 4 Cidofovir- Resistant Cowpox EC50 Monkeypox EC50 Camelpox EC50 A = <0.5 μM, A = <0.5 μM, A = <0.5 μM, Exam- B = 0.5 to <1.0 μM, B = 0.5 to <1.0 μM, B = 0.5 to <1.0 μM, ple C = 1.0 to <5 μM, C = 1.0 to <5 μM, C = 1.0 to <5 μM, Number D = ≧5 μM D = ≧5 μM D = ≧5 μM 1 A A A 2 A A A 3 A A A 15 A A A 24 B A A - The specificity of representative compounds for orthopox virus inhibition is reflected in that the fact that they do not inhibit the replication of unrelated viruses, including Pichinde virus, Rift Valley fever virus (strain MP12), respiratory syncytial virus and cytomegalovirus.
- Physico-Chemical Properties
- Appearance: ST-246 is a white to off-white powder.
- Melting Point: Approximately 196° C. by DSC.
- Permeability: The calculated log P is 2.94. Based on the partition coefficient, ST-246 is expected to have good permeability.
- Particle Size: The drug substance is micronized to improve its dissolution in the gastrointestinal fluids. The typical particle size of the micronized material is 50% less than 5 microns.
- Solubility: The solubility of ST-246 is low in water (0.026 mg/mL) and buffers of the gastric pH range. Surfactant increases its solubility slightly. ST-246 is very soluble in organic solvents. The solubility data are given in Table 5.
TABLE 5 Solubility of ST-246 in Various Diluents Diluent ST-246 Solubility (mg/mL) Phosphate Buffer, pH 6.8 0.00700 Water 0.0261 0.01N HCl 0.0375 1% Sodium Laurel Sulfate 0.0687 1% Tween 80 ® 0.102 Methanol 60.8 Ethanol 62.1 Acetonitrile 64.0 - Polymorphism: X-ray diffraction pattern indicated crystalline material and is similar for various batches. DSC shows sharp endotherms at 196° C. corresponding to the melting point. There are small endotherms between 115° C. and 189° C. The position of these endotherms varies in the two batches.
- Biopharmaceutical Classification: ST-246 is classified as BCS Class 2 due to its low solubility in solutions of gastric pH range and good permeability.
- Stability: Short-term forced degradation studies indicated that ST-246 has good stability in solid state, and in neutral, acidic, and basic (50:50 water/acetonitrile) solutions. Small amounts of degradation products were formed.
- The micronized ST-246 GMP batch 06-0503 16-04/04-24-0 1 (Batch # Micronized 5C020) intended for clinical trial was placed on a three-year stability program in the current container (amber glass bottles) according to International Conference for Harmonization (ICH) guidelines. Six-month data indicate that ST-246 is stable at 25° C./60% RH and 40° C./75% RH and light.
- There is no time or temperature dependent change in appearance, moisture, assay, impurities, and XRD. The DSC patterns at 2 months at 25° C./60% RH and 3 months at 40° C./75% RH show slight differences from that of the initial sample. From cyclic DSC and TGA analysis, it appears that the initial sample has a mixture of free and bound water as indicated by a broad endotherm at 115° C. (100-130° C.), and changes to bound water as indicated by a shift of this endotherm to 131-134° C. TGA shows loss of 2% water at 126° C.
- ST-246 may be manufactured in a process comprising the following four steps:
- Step 1: A solution of cycloheptatriene (1) and maleic anhydride (2) in anhydrous toluene is heated at 80° for 4 hours under a nitrogen atmosphere. After GC/MS analysis shows the reactions is complete, the reaction solution is cooled to room temperature and evaporated under reduced pressure. The resulting residue is recrystallized from tert-butyl methyl ether to afford the endo-isomer (3) as a white crystal.
- Step 2: To a solution of anhydrous hydrazine in anhydrous toluene is added methyl 4-(trifluoromethyl)benzoate (4). The reaction solution is heated at reflux for 18 hours under a nitrogen atmosphere. After cooling to 40-50° C., the solvent is evaporated under reduced pressure. The resulting solid is recrystallized from tert-butyl methyl ether to give hydrazide (5) as a white solid.
- Step 3: A mixture of the endo-isomer (3), the hydrazide (5), and ethanol is heated at reflux for 18 hours under a nitrogen atmosphere. The resulting solution is cooled to room temperature and concentrated in vacuo. The crude material (6) is used directly for the next step.
- Step 4: The crude material (6) is recrystallized from ethyl acetate and hexanes to obtain ST-246 (7) as a white solid. The material is dried for 48 hours at 40° C.
- For storage, the material may be packaged in amber glass bottles and stored at 2 to 8° C.
- The physical form for ST-246 is a white to off-white solid. The molecular formula is C19H15F3N2O3. The molecular weight is 376.33. The melting point is 196° C. by DSC. The solubility of ST-246 is low in water (0.026 mg/mL) and slightly in buffers of the gastric pH range. ST-246 is very soluble in organic solvents (60 mg/mL). The chemical structure is shown in Table 1, above (Example 1).
- The structure of ST-246 was elucidated using elemental analysis, infrared spectroscopy, ultraviolet spectroscopy, mass spectroscopy, proton nuclear magnetic resonance spectroscopy, and DSC. Elemental analysis was carried out on the following elements: carbon, hydrogen, fluorine, nitrogen, and oxygen. The results of the analysis are given below in Table 6. The elemental analysis results are consistent with ST-246 containing 0.235 moles of water.
TABLE 6 Elemental Analysis of ST-246 Expected With Expected As 0.235 Moles Element Anhydrous of Water Found Std. Dev. C 60.64 59.97 59.97 0.3 H 4.02 4.1 4.02 0.1 F 15.15 14.98 14.94 0.1 N 7.44 7.36 7.36 0.05 O 12.75 13.66 13.71 0.19 - Short-term forced degradation studies indicate that ST-246 has good stability in the solid state and in neutral, acidic, and basic (50:50 water/acetonitrile) solutions. Small amounts of degradation products were formed.
- A three-month long test of the stability of ST-246 has been conducted. Storage for three months at 40° C./75% RH and 25° C./65% RH conditions showed no change in the analytical results of any of the parameters tested.
- ST-246 can be formulated for oral administration in, for example, size 0 capsules containing either 25 mg or 200 mg ST-246. All inactive ingredients may be GRAS and USPLNF excipients. The manufacturing process may include wet granulation using a high shear mixer/granulator and filling into hard-gelatin capsules.
- Exemplary Components
- Microcrystalline cellulose, NF (Avicel PH 101)
- Lactose monohydrate, spray dried, NF, (Fast-flo)
- Croscarmellose sodium, NF (Ac-Di-Sol)
- Hydroxypropylmethyl cellulose, USP (Methocel E3)
- Sodium lauryl sulfate (SLS), NF
- Colloidal silicone dioxide, NF (Cab-0-Sil M5P)
- Magnesium stearate, NF (Non-bovine)
- Purified water, USP
- Hard gelatin capsule opaque white size 0
- Description and Quantitative Composition
- Suitable dosage forms include capsules containing various amounts of active ingredient. The quantitative composition of two exemplary dosage forms containing 25 or 200 mg of ST-246 are listed below in Table 6.
TABLE 6 Quantitative Composition for ST-246 Drug Product 200 mg strength 25 mg strength Ingredient Function mg/Capsule % w/w mg/Capsule % w/w ST-246a Active Ingredient 200.00 51.28 25.00 7.14 Microcrystalline cellulose, NFb Water Insoluble Diluent 88.60 22.72 144.76 41.36 Lactose monohydrate, NF Water soluble Diluent 33.15 8.50 119.0 34.0 Croscarmellose sodium, NFb Disintegrant 42.90 11.00 38.5 11.00 Colloidal silicon dioxide, NF Glidant 1.95 0.50 1.75 0.50 Hydroxypropyl methylcellulose, Binder 13.65 3.50 12.25 3.50 USP Sodium lauryl sulfate, NF Wetting Agent/Solubilizer 7.80 2.00 7.0 2.00 Purified waterc, USP Granulating solvent Magnesium stearate NF Lubricant 1.95 0.50 1.75 0.50 Tablet weight 390 100 350 100
aThe quantity of ST-246 may be adjusted based on the drug substance lot factor, which is calculated to reflect the purity along with the water and residual solvents content. A correspondingly reduced amount of microcrystalline cellulose will be adjusted to maintain the same capsule weight.
bMicrocrystalline cellulose and croscarmellose sodium are added as intragranular and extragranular excipients.
cRemoved during processing.
- Typical Batch Formula
- Batch sizes may vary. Formulas for typical batch sizes are listed below in Table 7.
TABLE 7 200 mg Capsules 25 mg Capsules Excipients g per 2400 g Batch g per 800 g Batch ST-246, micronized* 1230.9 57.14 Microcrystalline cellulose, 545.1 330.88 NF (Avicel PH101) Lactose monohydrate, 204.0 272.00 NF (Fast Flo) Croscarmellose sodium, 264.0 88.00 NF (Ac-Di-Sol) Hydroxypropylmethyl 84.0 28.00 cellulose, USP (Methocel E3) Sodium lauryl sulfate, NF 48.0 16.00 Colloidal silicone dioxide, 12.0 4.00 NF (Cab-0-Sil M5P) Magnesium stearate, 12.0 4.00 NF (Non-bovine) Purified water**, USP Total Weight 2400 800 Capsules, empty, hard 6000 2285 gelatin, size 0, white/white Capsules Capsules opaque
*The quantity of ST-246 may be adjusted based on the drug substance lot factor, which is calculated to reflect the purity along with the water and residual solvents content. A corresponding reduced amount of microcrystalline cellulose will be adjusted to maintain the same fill weight per capsule.
**Removed during drying.
- Step-Wise Manufacturing Procedure
- A stepwise process for the manufacture of ST-246 25 mg and 200 mg capsules, is listed below.
- 1. Dissolve sodium lauryl sulfate and hydroxypropylmethyl cellulose in purified water.
- 2. Sift through 20-mesh screen and mix ST-246, microcrystalline cellulose, croscarmellose sodium, lactose, and colloidal silicone dioxide at slow speed in high shear mixer.
- 3. Add sodium lauryl sulfate and hydroxypropylmethyl cellulose solution while mixing.
- 4. Mix at slow speed after addition of solution.
- 5. Add more purified water if needed and mix.
- 6. Dry in the fluid bed dryer.
- 7. Pass the dried granulation through #30 mesh screen. Pass the granulation remaining on top of #30 mesh screen using comil.
- 8. Weigh the granulation and calculate quantities of the extragranular excipient.
- 9. Add milled granulation into a V-blender and add croscarmellose sodium and microcrystalline cellulose (pre-screened through 20-mesh) to the blender, and mix. Take a portion of the blend and mix with magnesium stearate, add to the blender and mix.
- 10. Fill into capsules.
- One of ordinary skill will readily be able to modify this process in order to accommodate different amounts of ST-246 per dose as required.
- Although the present invention has been described and exemplified in terms of certain preferred embodiments, other embodiments will be apparent to those skilled in the art. The invention is, therefore, not limited to the particular embodiments described and exemplified, but is capable of modification or variation without departing from the spirit of the invention, the full scope of which is delineated by the appended claims.
Claims (12)
Priority Applications (21)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP07755857A EP2148860A4 (en) | 2007-04-23 | 2007-04-23 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| CA2866037A CA2866037C (en) | 2007-04-23 | 2007-04-23 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| JP2010506148A JP2010535705A (en) | 2007-04-23 | 2007-04-23 | Chemicals, compositions and methods for the treatment and prevention of orthopoxvirus infection and related diseases |
| MX2014015012A MX348481B (en) | 2007-04-23 | 2007-04-23 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases. |
| MX2009011533A MX2009011533A (en) | 2007-04-23 | 2007-04-23 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases. |
| CA2966466A CA2966466C (en) | 2007-04-23 | 2007-04-23 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US11/785,998 US8039504B2 (en) | 2003-06-20 | 2007-04-23 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| CN200780053386A CN101702904A (en) | 2007-04-23 | 2007-04-23 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| CN201510088562.0A CN104758281A (en) | 2007-04-23 | 2007-04-23 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| CA2685153A CA2685153C (en) | 2007-04-23 | 2007-04-23 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| PCT/US2007/009751 WO2008130348A1 (en) | 2007-04-23 | 2007-04-23 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| EP15195740.4A EP3006425A1 (en) | 2007-04-23 | 2007-04-23 | Pharmaceutical composition for use in the treatment and prevention of orthopoxvirus infections and associated diseases |
| AU2007351866A AU2007351866C1 (en) | 2003-06-20 | 2007-04-23 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| MX2017007471A MX363189B (en) | 2007-04-23 | 2007-04-23 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases. |
| IL201736A IL201736A (en) | 2007-04-23 | 2009-10-25 | Compositions comprising cyclic acylhydrazide derivatives and a dosage unit comprising the same |
| US13/194,437 US8530509B2 (en) | 2003-06-20 | 2011-07-29 | Compounds, compositions and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US13/966,392 US8802714B2 (en) | 2003-06-20 | 2013-08-14 | Compounds, compositions and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US14/323,587 US9045418B2 (en) | 2003-06-20 | 2014-07-03 | Compounds, compositions and methods for treatment and prevention of Orthopoxvirus infections and associated diseases |
| IL242666A IL242666B (en) | 2007-04-23 | 2015-11-19 | Process for producing 4-trifluoromethyl-n-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f] isoindol-2(lh)-yl)-benzamide (st-246) |
| IL24266515A IL242665B (en) | 2007-04-23 | 2015-11-19 | A process for producing anti-tricyclo[3.2.2.0]non-6-ene-endo-8,endo-9-dicarboxylic acid anhydride |
| IL269370A IL269370B (en) | 2007-04-23 | 2019-09-15 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US48018203P | 2003-06-20 | 2003-06-20 | |
| PCT/US2004/019552 WO2004112718A2 (en) | 2003-06-20 | 2004-06-18 | Compounds, compositions and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US10/561,153 US7737168B2 (en) | 2003-06-20 | 2004-06-18 | Compounds, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US11/785,998 US8039504B2 (en) | 2003-06-20 | 2007-04-23 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
Related Parent Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2004/019552 Continuation-In-Part WO2004112718A2 (en) | 2003-06-20 | 2004-06-18 | Compounds, compositions and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US10/561,153 Continuation-In-Part US7737168B2 (en) | 2003-06-20 | 2004-06-18 | Compounds, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US11/561,153 Continuation-In-Part US20070094707A1 (en) | 2000-02-01 | 2006-11-17 | Rule Based Security Policy Enforcement |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/194,437 Continuation-In-Part US8530509B2 (en) | 2003-06-20 | 2011-07-29 | Compounds, compositions and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20070287735A1 true US20070287735A1 (en) | 2007-12-13 |
| US8039504B2 US8039504B2 (en) | 2011-10-18 |
Family
ID=39876302
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/785,998 Active 2027-07-23 US8039504B2 (en) | 2003-06-20 | 2007-04-23 | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US8039504B2 (en) |
| EP (2) | EP3006425A1 (en) |
| JP (1) | JP2010535705A (en) |
| CN (2) | CN104758281A (en) |
| AU (1) | AU2007351866C1 (en) |
| CA (3) | CA2966466C (en) |
| IL (4) | IL201736A (en) |
| MX (3) | MX348481B (en) |
| WO (1) | WO2008130348A1 (en) |
Cited By (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080004452A1 (en) * | 2003-06-20 | 2008-01-03 | Siga Technologies, Inc. | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US20080300265A1 (en) * | 2006-03-02 | 2008-12-04 | Siga Technologies Inc. | Antiviral drugs for treatment of arenavrus infection |
| US20090180980A1 (en) * | 2006-02-01 | 2009-07-16 | Siga Technologies, Inc. | Anti-Arenaviral Compunds |
| US20090203675A1 (en) * | 2004-12-06 | 2009-08-13 | Siga Technologies, Inc. | Sulfonyl Semicarbazides, Semicarbazides and Ureas, Pharmaceutical Compositions Thereof, and Methods for Treating Hemorrhagic Fever Viruses, Including Infections Associated with Arena Viruses |
| US20100167180A1 (en) * | 2007-04-06 | 2010-07-01 | Liangheng Xu | Photosensitive Film for Holographic Recording and Production Method Thereof |
| US20100189685A1 (en) * | 2007-05-23 | 2010-07-29 | Byrd Chelsea M | Antiviral Drugs for Treatment or Prevention of Dengue Infection |
| US20100227892A1 (en) * | 2003-06-20 | 2010-09-09 | Siga Technologies Inc. | Compounds, Compositions and Methods for Treatment and Prevention of Orthopoxvirus Infections and Associated Diseases |
| US7872037B2 (en) | 2006-03-02 | 2011-01-18 | Siga Technologies, Inc. | Antiviral drugs for treatment of arenavirus infection |
| US7994221B2 (en) | 2004-12-06 | 2011-08-09 | Siga Technologies, Inc. | Sulfonyl semicarbazides, carbonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arenaviruses |
| WO2012018810A1 (en) * | 2010-08-05 | 2012-02-09 | Siga Technologies, Inc. | St-246 liquid formulations and methods |
| US8148428B2 (en) | 2006-03-02 | 2012-04-03 | Siga Technologies, Inc. | Antiviral drugs for treatment of arenavirus infection |
| US8410149B2 (en) | 2004-12-06 | 2013-04-02 | Siga Technologies Inc. | Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arenaviruses |
| US8461177B2 (en) | 2007-08-27 | 2013-06-11 | Siga Technologies, Inc. | Antiviral drugs for treatment of arenavirus infection |
| WO2014028545A1 (en) * | 2012-08-16 | 2014-02-20 | Siga Technologies, Inc. | Methods of preparing tecovirimat |
| US8791110B2 (en) | 2006-02-01 | 2014-07-29 | Siga Technologies, Inc. | Anti-arenaviral compounds |
| US8871746B2 (en) | 2006-03-02 | 2014-10-28 | Kineta Four, LLC | Antiviral drugs for treatment of arenavirus infection |
| WO2018218093A1 (en) * | 2017-05-26 | 2018-11-29 | Medimmune, Llc | Method and molecules |
| CN112203696A (en) * | 2018-05-25 | 2021-01-08 | 免疫医疗有限公司 | Pyrrolobenzodiazepine conjugates |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8530509B2 (en) | 2003-06-20 | 2013-09-10 | Siga Technologies, Inc. | Compounds, compositions and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| JP2010535705A (en) | 2007-04-23 | 2010-11-25 | シガ テクノロジーズ,インコーポレーテッド | Chemicals, compositions and methods for the treatment and prevention of orthopoxvirus infection and related diseases |
| CN101747258B (en) * | 2008-12-04 | 2013-12-25 | 中国人民解放军军事医学科学院毒物药物研究所 | N-[(3aR, 4R, 4aR, 5aS, 6S, 6aS)-1,3-dioxo-3, 3a, 4, 4a 5, 5a, 6, 6a-octa-4,6- ethenylene cyclopropane[f] Isoindoline-2(1H)-base]-4-(trifluoromethyl) benzamide-hydrate and medical purpose thereof |
| KR20160062208A (en) * | 2010-03-23 | 2016-06-01 | 시가 테크놀로지스, 인크. | Polymorphic forms st-246 and methods of preparation |
| CN101912389A (en) * | 2010-08-09 | 2010-12-15 | 中国人民解放军军事医学科学院微生物流行病研究所 | Pharmaceutical composition containing ST-246 and preparation method and application thereof |
| CN101906064A (en) * | 2010-08-09 | 2010-12-08 | 中国人民解放军军事医学科学院微生物流行病研究所 | Preparation method of ST-246 stereoisomer |
| AU2014353235B2 (en) * | 2013-11-19 | 2019-05-09 | Siga Technologies, Inc. | Rehydration of micronized tecovirimat monohydrate |
| CN114150005B (en) * | 2022-02-09 | 2022-04-22 | 广州恩宝生物医药科技有限公司 | Adenovirus vector vaccine for prevention of SARS-CoV-2 Oncuronjorn strain |
| CN116730995A (en) * | 2022-08-12 | 2023-09-12 | 深圳微芯生物科技股份有限公司 | Hydrazide compound, preparation method and application thereof |
| CN116253674A (en) * | 2023-02-06 | 2023-06-13 | 青岛增益安生物科技有限公司 | Method for preparing tecovirime |
| CN117582436A (en) * | 2023-08-31 | 2024-02-23 | 甫康(上海)健康科技有限责任公司 | Iminoacyl hydrazides, compositions containing same and uses thereof |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2906755A (en) * | 1956-11-16 | 1959-09-29 | Schenley Ind Inc | N-acyl derivatives of alpha-pyridine carboxylic acid hydrazide |
| US4029666A (en) * | 1967-08-09 | 1977-06-14 | Hoechst Aktiengesellschaft | Benzenesulfonyl-ureas and process for preparing them |
| US4061763A (en) * | 1975-05-09 | 1977-12-06 | Merck & Co., Inc. | Tricyclicdicarboximides |
| US4173646A (en) * | 1974-10-04 | 1979-11-06 | Merck & Co., Inc. | Tricyclicdicarboximides |
| US5068356A (en) * | 1990-02-20 | 1991-11-26 | Atochem North America, Inc. | Hindered phenolic n-(amido)imides |
| US5441976A (en) * | 1990-06-08 | 1995-08-15 | Aktiebolaget Astra | Pharmacological use of certain cystine derivatives |
| US5994344A (en) * | 1996-03-28 | 1999-11-30 | Glaxo Wellcome Inc. | Pyrrolopyrrolone derivatives as inhibitors of neutrophil elastase |
| US20070254934A1 (en) * | 2006-03-02 | 2007-11-01 | Hruby Dennis E | Antiviral drugs for treatment of arenavirus infection |
| US20080004452A1 (en) * | 2003-06-20 | 2008-01-03 | Siga Technologies, Inc. | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US20080103181A9 (en) * | 2003-06-20 | 2008-05-01 | Siga Technologies, Inc. | Compounds, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2256702A (en) * | 2000-12-01 | 2002-06-11 | Kyowa Hakko Kogyo Kk | Composition improved in solubility or oral absorbability |
| US6433016B1 (en) | 2001-01-12 | 2002-08-13 | Vassil Stefanov Georgiev | Drugs for treating viral infections |
| US6596771B2 (en) | 2001-01-12 | 2003-07-22 | Schering Corporation | Drugs for treating viral infections |
| US20040087548A1 (en) | 2001-02-27 | 2004-05-06 | Salvati Mark E. | Fused cyclic succinimide compounds and analogs thereof, modulators of nuclear hormone receptor function |
| WO2004112718A2 (en) * | 2003-06-20 | 2004-12-29 | Siga Technologies, Inc. | Compounds, compositions and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| EP1698349A4 (en) * | 2003-12-25 | 2009-12-16 | Takeda Pharmaceutical | Method of improving suitability for granulation |
| JP2010535705A (en) | 2007-04-23 | 2010-11-25 | シガ テクノロジーズ,インコーポレーテッド | Chemicals, compositions and methods for the treatment and prevention of orthopoxvirus infection and related diseases |
-
2007
- 2007-04-23 JP JP2010506148A patent/JP2010535705A/en active Pending
- 2007-04-23 WO PCT/US2007/009751 patent/WO2008130348A1/en active Application Filing
- 2007-04-23 EP EP15195740.4A patent/EP3006425A1/en not_active Withdrawn
- 2007-04-23 US US11/785,998 patent/US8039504B2/en active Active
- 2007-04-23 EP EP07755857A patent/EP2148860A4/en not_active Withdrawn
- 2007-04-23 MX MX2014015012A patent/MX348481B/en unknown
- 2007-04-23 CA CA2966466A patent/CA2966466C/en active Active
- 2007-04-23 CN CN201510088562.0A patent/CN104758281A/en active Pending
- 2007-04-23 CA CA2866037A patent/CA2866037C/en active Active
- 2007-04-23 CN CN200780053386A patent/CN101702904A/en active Pending
- 2007-04-23 AU AU2007351866A patent/AU2007351866C1/en active Active
- 2007-04-23 CA CA2685153A patent/CA2685153C/en active Active
- 2007-04-23 MX MX2009011533A patent/MX2009011533A/en active IP Right Grant
- 2007-04-23 MX MX2017007471A patent/MX363189B/en unknown
-
2009
- 2009-10-25 IL IL201736A patent/IL201736A/en active IP Right Grant
-
2015
- 2015-11-19 IL IL242666A patent/IL242666B/en active IP Right Grant
- 2015-11-19 IL IL24266515A patent/IL242665B/en active IP Right Grant
-
2019
- 2019-09-15 IL IL269370A patent/IL269370B/en active IP Right Grant
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2906755A (en) * | 1956-11-16 | 1959-09-29 | Schenley Ind Inc | N-acyl derivatives of alpha-pyridine carboxylic acid hydrazide |
| US4029666A (en) * | 1967-08-09 | 1977-06-14 | Hoechst Aktiengesellschaft | Benzenesulfonyl-ureas and process for preparing them |
| US4173646A (en) * | 1974-10-04 | 1979-11-06 | Merck & Co., Inc. | Tricyclicdicarboximides |
| US4061763A (en) * | 1975-05-09 | 1977-12-06 | Merck & Co., Inc. | Tricyclicdicarboximides |
| US5068356A (en) * | 1990-02-20 | 1991-11-26 | Atochem North America, Inc. | Hindered phenolic n-(amido)imides |
| US5441976A (en) * | 1990-06-08 | 1995-08-15 | Aktiebolaget Astra | Pharmacological use of certain cystine derivatives |
| US5994344A (en) * | 1996-03-28 | 1999-11-30 | Glaxo Wellcome Inc. | Pyrrolopyrrolone derivatives as inhibitors of neutrophil elastase |
| US20080004452A1 (en) * | 2003-06-20 | 2008-01-03 | Siga Technologies, Inc. | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US20080103181A9 (en) * | 2003-06-20 | 2008-05-01 | Siga Technologies, Inc. | Compounds, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US7687641B2 (en) * | 2003-06-20 | 2010-03-30 | Siga Technologies, Inc. | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US20070254934A1 (en) * | 2006-03-02 | 2007-11-01 | Hruby Dennis E | Antiviral drugs for treatment of arenavirus infection |
Cited By (56)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100227892A1 (en) * | 2003-06-20 | 2010-09-09 | Siga Technologies Inc. | Compounds, Compositions and Methods for Treatment and Prevention of Orthopoxvirus Infections and Associated Diseases |
| US20080004452A1 (en) * | 2003-06-20 | 2008-01-03 | Siga Technologies, Inc. | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US8124643B2 (en) | 2003-06-20 | 2012-02-28 | Siga Technologies, Inc. | Compounds, compositions and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US7956197B2 (en) | 2003-06-20 | 2011-06-07 | Siga Technologies, Inc. | Compounds, compositions and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US7687641B2 (en) | 2003-06-20 | 2010-03-30 | Siga Technologies, Inc. | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
| US20110009456A1 (en) * | 2003-06-20 | 2011-01-13 | Siga Technologies Inc. | Compounds, Compositions and Methods for Treatment and Prevention of Orthopoxvirus Infections and Associated Diseases |
| US9067873B2 (en) | 2004-12-06 | 2015-06-30 | Kineta Four, LLC | Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arenaviruses |
| US7994221B2 (en) | 2004-12-06 | 2011-08-09 | Siga Technologies, Inc. | Sulfonyl semicarbazides, carbonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arenaviruses |
| US8642596B2 (en) | 2004-12-06 | 2014-02-04 | Siga Technologies, Inc. | Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arena viruses |
| US9115065B2 (en) | 2004-12-06 | 2015-08-25 | Kineta, Inc. | Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with Arenaviruses |
| US20090203675A1 (en) * | 2004-12-06 | 2009-08-13 | Siga Technologies, Inc. | Sulfonyl Semicarbazides, Semicarbazides and Ureas, Pharmaceutical Compositions Thereof, and Methods for Treating Hemorrhagic Fever Viruses, Including Infections Associated with Arena Viruses |
| US8410149B2 (en) | 2004-12-06 | 2013-04-02 | Siga Technologies Inc. | Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arenaviruses |
| US8664274B2 (en) | 2004-12-06 | 2014-03-04 | Siga Technologies, Inc. | Sulfonyl semicarbazides, semicarbazides and ureas, pharmaceutical compositions thereof, and methods for treating hemorrhagic fever viruses, including infections associated with arena viruses |
| US8106058B2 (en) | 2006-02-01 | 2012-01-31 | Siga Technologies, Inc. | Anti-arenaviral compounds |
| US8791110B2 (en) | 2006-02-01 | 2014-07-29 | Siga Technologies, Inc. | Anti-arenaviral compounds |
| US8518951B2 (en) | 2006-02-01 | 2013-08-27 | Siga Technologies, Inc. | Anti-arenaviral compounds |
| US20090180980A1 (en) * | 2006-02-01 | 2009-07-16 | Siga Technologies, Inc. | Anti-Arenaviral Compunds |
| US20080300265A1 (en) * | 2006-03-02 | 2008-12-04 | Siga Technologies Inc. | Antiviral drugs for treatment of arenavrus infection |
| US20110172281A1 (en) * | 2006-03-02 | 2011-07-14 | Siga Technologies Inc. | Anti-Viral Drugs for Treatment of Arenavirus Infection |
| US8148428B2 (en) | 2006-03-02 | 2012-04-03 | Siga Technologies, Inc. | Antiviral drugs for treatment of arenavirus infection |
| US7977365B2 (en) | 2006-03-02 | 2011-07-12 | Siga Technologies, Inc. | Antiviral drugs for treatment of arenavirus infection |
| US9199920B2 (en) | 2006-03-02 | 2015-12-01 | Kineta Four, LLC | Antiviral drugs for treatment of arenavirus infection |
| US8492434B2 (en) | 2006-03-02 | 2013-07-23 | Siga Technologies Inc. | Antiviral drugs for treatment of arenavirus infection |
| US7872037B2 (en) | 2006-03-02 | 2011-01-18 | Siga Technologies, Inc. | Antiviral drugs for treatment of arenavirus infection |
| US8871746B2 (en) | 2006-03-02 | 2014-10-28 | Kineta Four, LLC | Antiviral drugs for treatment of arenavirus infection |
| US8629170B2 (en) | 2006-03-02 | 2014-01-14 | Siga Technologies, Inc. | Anti-viral drugs for treatment of arenavirus infection |
| US20100167180A1 (en) * | 2007-04-06 | 2010-07-01 | Liangheng Xu | Photosensitive Film for Holographic Recording and Production Method Thereof |
| US20100189685A1 (en) * | 2007-05-23 | 2010-07-29 | Byrd Chelsea M | Antiviral Drugs for Treatment or Prevention of Dengue Infection |
| US8518960B2 (en) | 2007-05-23 | 2013-08-27 | Siga Technologies, Inc. | Antiviral drugs for treatment or prevention of dengue infection |
| US9353051B2 (en) | 2007-05-23 | 2016-05-31 | Siga Technologies, Inc. | Antiviral drugs for treatment or prevention of dengue infection |
| US8754082B2 (en) | 2007-08-27 | 2014-06-17 | Siga Technologies, Inc. | Antiviral drugs for treatment of Arenavirus infection |
| US9096597B2 (en) | 2007-08-27 | 2015-08-04 | Kineta Four, LLC | Antiviral drugs for treatment of Arenavirus infection |
| US8461177B2 (en) | 2007-08-27 | 2013-06-11 | Siga Technologies, Inc. | Antiviral drugs for treatment of arenavirus infection |
| KR20130135836A (en) * | 2010-08-05 | 2013-12-11 | 시가 테크놀로지스, 인크. | St-246 liquid formulations and methods |
| US10124071B2 (en) | 2010-08-05 | 2018-11-13 | Siga Technologies, Inc. | ST-246 liquid formulations and methods |
| AU2011285871A8 (en) * | 2010-08-05 | 2015-05-14 | Siga Technologies, Inc. | ST-246 liquid formulations and methods |
| CN103281898A (en) * | 2010-08-05 | 2013-09-04 | 西佳科技股份有限公司 | St-246 liquid formulations and methods |
| AU2011285871B2 (en) * | 2010-08-05 | 2015-04-23 | Siga Technologies, Inc. | ST-246 liquid formulations and methods |
| US9233097B2 (en) | 2010-08-05 | 2016-01-12 | Siga Technologies, Inc. | ST-246 liquid formulations |
| EP2600715A4 (en) * | 2010-08-05 | 2016-04-20 | Siga Technologies Inc | St-246 liquid formulations and methods |
| WO2012018810A1 (en) * | 2010-08-05 | 2012-02-09 | Siga Technologies, Inc. | St-246 liquid formulations and methods |
| CN106074370A (en) * | 2010-08-05 | 2016-11-09 | 西佳科技股份有限公司 | ST 246 liquid formulation and method |
| US10864282B2 (en) | 2010-08-05 | 2020-12-15 | Siga Technologies Inc. | ST-246 liquid formulations and methods |
| US10576165B2 (en) | 2010-08-05 | 2020-03-03 | Siga Technologies Inc. | ST-246 liquid formulations and methods |
| US9907859B2 (en) | 2010-08-05 | 2018-03-06 | Siga Technologies, Inc. | ST-246 liquid formulations and methods |
| KR101868117B1 (en) * | 2010-08-05 | 2018-06-18 | 시가 테크놀로지스, 인크. | St-246 liquid formulations and methods |
| US9862683B2 (en) | 2012-08-16 | 2018-01-09 | Siga Technologies, Inc. | Methods of preparing Tecovirimat |
| US10029985B2 (en) | 2012-08-16 | 2018-07-24 | Siga Technologies Inc. | Methods of preparing Tecovirimat |
| US10155723B2 (en) | 2012-08-16 | 2018-12-18 | Siga Technologies, Inc. | Methods of preparing tecovirimat |
| WO2014028545A1 (en) * | 2012-08-16 | 2014-02-20 | Siga Technologies, Inc. | Methods of preparing tecovirimat |
| US10662155B2 (en) | 2012-08-16 | 2020-05-26 | Siga Technologies Inc. | Methods of preparing tecovirimat |
| US9546137B2 (en) | 2012-08-16 | 2017-01-17 | Siga Technologies Inc. | Methods of preparing Tecovirimat |
| WO2018218093A1 (en) * | 2017-05-26 | 2018-11-29 | Medimmune, Llc | Method and molecules |
| CN110650947A (en) * | 2017-05-26 | 2020-01-03 | 免疫医疗有限责任公司 | Methods and molecules |
| US11759526B2 (en) | 2017-05-26 | 2023-09-19 | Medimmune, Llc | Method and molecules |
| CN112203696A (en) * | 2018-05-25 | 2021-01-08 | 免疫医疗有限公司 | Pyrrolobenzodiazepine conjugates |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2966466C (en) | 2020-08-25 |
| IL242666B (en) | 2018-08-30 |
| MX348481B (en) | 2017-06-15 |
| IL201736A0 (en) | 2010-06-16 |
| CA2685153A1 (en) | 2008-10-30 |
| CA2866037C (en) | 2017-05-16 |
| JP2010535705A (en) | 2010-11-25 |
| MX363189B (en) | 2019-03-14 |
| WO2008130348A1 (en) | 2008-10-30 |
| IL201736A (en) | 2016-06-30 |
| CA2685153C (en) | 2014-12-16 |
| IL269370B (en) | 2020-08-31 |
| US8039504B2 (en) | 2011-10-18 |
| IL269370A (en) | 2019-11-28 |
| EP3006425A1 (en) | 2016-04-13 |
| IL242665B (en) | 2019-10-31 |
| EP2148860A1 (en) | 2010-02-03 |
| CA2966466A1 (en) | 2008-10-30 |
| AU2007351866B2 (en) | 2012-09-27 |
| MX2009011533A (en) | 2010-04-07 |
| AU2007351866A1 (en) | 2008-10-30 |
| EP2148860A4 (en) | 2010-12-22 |
| CN104758281A (en) | 2015-07-08 |
| AU2007351866C1 (en) | 2013-07-25 |
| CA2866037A1 (en) | 2008-10-30 |
| CN101702904A (en) | 2010-05-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8039504B2 (en) | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases | |
| US7687641B2 (en) | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases | |
| US9045418B2 (en) | Compounds, compositions and methods for treatment and prevention of Orthopoxvirus infections and associated diseases | |
| US8124643B2 (en) | Compounds, compositions and methods for treatment and prevention of orthopoxvirus infections and associated diseases | |
| JP5657489B2 (en) | Compounds, compositions and methods for treating and preventing orthopoxvirus infection and related diseases | |
| AU2012268859B2 (en) | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases | |
| AU2016208315A1 (en) | Chemicals, compositions, and methods for treatment and prevention of orthopoxvirus infections and associated diseases |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: SIGA TECHNOLOGIES, INC., NEW YORK Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:JORDAN, ROBERT F.;BAILEY, THOMAS R.;RIPPIN, SUSAN R.;AND OTHERS;REEL/FRAME:019572/0556;SIGNING DATES FROM 20070622 TO 20070626 Owner name: SIGA TECHNOLOGIES, INC., NEW YORK Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:JORDAN, ROBERT F.;BAILEY, THOMAS R.;RIPPIN, SUSAN R.;AND OTHERS;SIGNING DATES FROM 20070622 TO 20070626;REEL/FRAME:019572/0556 |
|
| STCF | Information on status: patent grant |
Free format text: PATENTED CASE |
|
| AS | Assignment |
Owner name: NATIONAL INSTITUTES OF HEALTH (NIH), U.S. DEPT. OF Free format text: CONFIRMATORY LICENSE;ASSIGNOR:SIGA TECHNOLOGIES, INC.;REEL/FRAME:027359/0048 Effective date: 20111107 |
|
| FPAY | Fee payment |
Year of fee payment: 4 |
|
| AS | Assignment |
Owner name: CORTLAND CAPITAL MARKET SERVICES, LLC, ILLINOIS Free format text: SECURITY INTEREST;ASSIGNOR:SIGA TECHNOLOGIES, INC.;REEL/FRAME:040349/0219 Effective date: 20161116 |
|
| MAFP | Maintenance fee payment |
Free format text: PAYMENT OF MAINTENANCE FEE, 8TH YR, SMALL ENTITY (ORIGINAL EVENT CODE: M2552); ENTITY STATUS OF PATENT OWNER: SMALL ENTITY Year of fee payment: 8 |
|
| AS | Assignment |
Owner name: SIGA TECHNOLOGIES, INC., NEW YORK Free format text: RELEASE OF SECURITY INTEREST IN INTELLECTUAL PROPERTY AT REEL/FRAME NO. 40349/0219;ASSIGNOR:CORTLAND CAPITAL MARKET SERVICES LLC;REEL/FRAME:052163/0014 Effective date: 20200312 |
|
| MAFP | Maintenance fee payment |
Free format text: PAYMENT OF MAINTENANCE FEE, 12TH YR, SMALL ENTITY (ORIGINAL EVENT CODE: M2553); ENTITY STATUS OF PATENT OWNER: SMALL ENTITY Year of fee payment: 12 |